-
PDF
- Split View
-
Views
-
Cite
Cite
Benjamin W.P. Rossi, Sam Booth, Norman England, Neil J. Smart, Ian R. Daniels, Mucinous adenocarcinoma of the umbilicus 8 years following anterior resection for villous adenoma of the rectum, Journal of Surgical Case Reports, Volume 2014, Issue 1, January 2014, rjt098, https://doi.org/10.1093/jscr/rjt098
Close - Share Icon Share
Abstract
We present the case of an 80-year-old retired consultant histopathologist who presented to us with a malignant umbilical mass 8years following resection of a sigmoid adenoma. The report details initial investigation and management of the umbilical mass and the subsequently discovered pelvic recurrence. Our conclusions of its origin, as a malignant transformation due to seeding of the original sigmoid adenoma, show the slow progression of some colorectal tumours; and the importance of obtaining a complete specimen intra-operatively.
INTRODUCTION
This is a case of an 80-year-old retired consultant histopathologist who presented with an umbilical mass following resection of a sigmoid adenoma 8years previously.
PubMed was searched to look for similar cases. On searching the literature, we have found little evidence of any similar cases of benign adenoma undergoing malignant change leading to metastatic recurrence in the manner we have described.
CASE REPORT
The patient, a retired consultant histopathologist, was referred in August 2008 with a hard para-umbilical nodule, which was tethered to the abdominal wall; this was proximal to the site of a previous laparotomy scar from an anterior resection which he underwent in 2001 for villous adenoma at the recto-sigmoid junction. He had been well since and annual colonoscopies showed only mild diverticular disease, the last of which had been in 2007.
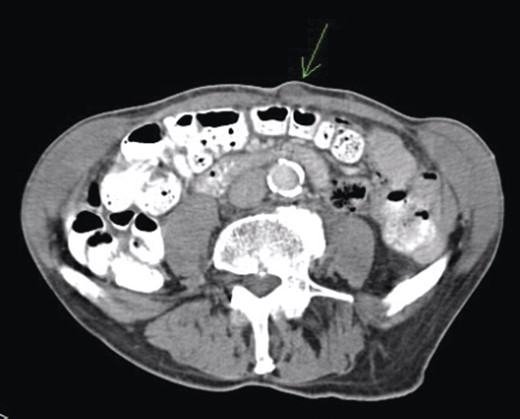
Given his previous colorectal disease, a repeat colonoscopy and computed tomography (CT) scan (Fig. 1) were arranged and the carcinoembryonic antigen (CEA) level was checked. Colonoscopy showed some thickening of the anastomosis but was otherwise unremarkable, and a CT scan showed no significant findings. CEA was raised at 11.2; therefore, excision of the lesion was done in January 2009 and following histopathological analysis, a positron emission tomography (PET) scan was arranged.
The histology from the lesion showed a well-differentiated mucinous adenocarcinoma consistent with colorectal origin. As the margin of the sample contained tumour, a complete excision of the umbilicus was performed in April 2009. His previous histology from 2001 was reviewed, both by the patient and a local histopathologist and confirmed to be villous adenoma with low-grade dysplasia and revealed that nearly the entire sample had been viewed to confirm no malignancy; however, the specimen was perforated at the time of operation but with clear resection margins. A PET scan in March 2009 showed no disseminated or residual disease.
In October 2009, a magnetic resonance imaging (MRI) scan of his spine was arranged due to leg pain thought to be of neurological origin. This did, indeed, showed marked L5 and S1 spinal stenosis; however, it also showed an irregular mass in the left side of the pelvis anterior to the sacrum and abutting the distal rectum and the emerging left S1 nerve root on the anterior surface of the sacrum. Overall, the dimensions were 3.6 × 4.3 × 3.8 cm. There was a similar area of soft tissue on the previous CT of September 2008. The possibility of a further slow-growing rectal recurrence was raised. This was discussed at multidisciplinary team meeting and as the mass was cold on PET scan in March 2009, it was decided to repeat the PET scan, which was done in February 2010 and November 2010, and the mass again showed no uptake.
Colonoscopy was repeated in September 2010 and this showed an enlarged mass which was palpable through the rectal wall. This began to cause symptoms of urinary retention and after a number of episodes of retention and re-catheterization, a suprapubic catheter was inserted in February 2012.
In July 2012, a follow-up MRI was done as surveillance for the mass, and this showed that the mass had extended into the left S1 neural foramen and another similar high signal area was seen to the left of the lower rectum and his CEA has continued to rise. After much discussion and in view of age and co-morbidity, together with the involvement of the pelvic sidewall, an R0 resection was not possible.
DISCUSSION
Given the extent of investigation to rule out another source of malignancy, the conclusion can be drawn that this is indeed a recurrence of the original villous adenoma that has undergone malignant change. This is a rare case that we believe is due to intra-operative seeding of tumour cells, as the original specimen was perforated. The time for malignant transformation from adenoma to carcinoma is indicative of the slow progression in some colorectal cancers [1, 2], presumably with mucin production (Figs 2 and 3). This case highlights the importance of obtaining a complete specimen with clear resection margins [3–5].
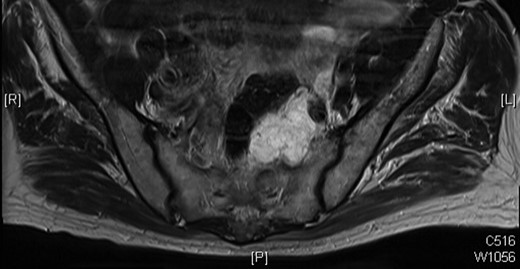
MRI scan of 2009 showing a mucinous mass in the left side of the pelvis below the anastomosis from his original sigmoid colectomy.
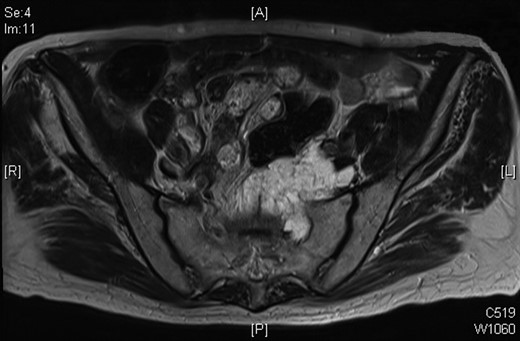
Progression of the mucinous mass on MRI in 2012. On repeated PET scanning, the mass shows minimal cellular activity.



