-
PDF
- Split View
-
Views
-
Cite
Cite
Kanellos Gesakis, Grigorios Tanos, Obi Onyekwelu, Anastasios Gaitis, Laxminarayan Gudur, Anil Agarwal, Primary spindle cell sarcoma of the breast masquerading as necrotizing fasciitis, Journal of Surgical Case Reports, Volume 2014, Issue 1, January 2014, rjt096, https://doi.org/10.1093/jscr/rjt096
Close - Share Icon Share
Abstract
Breast sarcomas are rare neoplasms arising from the few epithelial elements of the gland. It represents much <1% of all breast cancer. Of the heterogeneous group of sarcomas, the more common subtypes include spindle cell sarcoma. The main risk factor for the development of breast sarcomas is previous radiation therapy following breast-conservation surgery for breast cancer or non-Hodgkin's lymphoma. We report on an idiopathic presentation of spindle cell sarcoma in an otherwise healthy middle-aged woman. An emphasis is made on the rare occurrence of lymphatic metastasis. We discuss our recommended management strategy with particular reference to the benefit of multidisciplinary team decision-making.
INTRODUCTION
Most invasive breast neoplasms are epithelial tumours. Mesenchymal breast neoplasms are rare. Sarcoma is a heterogeneous neoplasm arising from mesenchymal cells. Breast sarcomas are rare neoplasms derived from non- epithelial elements of the gland and represent <1% of all breast cancers [1] estimated as 45 new cases per 10 million women. Most cases of breast sarcoma are secondary to radiotherapy due to increasing use of breast radiation after breast-conserving treatment. About 1 of 300 patients receiving radiotherapy for breast cancer can be expected to develop sarcoma [1]. Only a few hundred cases of breast sarcomas have been reported in the literature making the diagnosis difficult. We present a rare case of lymphatic metastases of primary spindle cell sarcoma of the breast.
Sarcomas can occur anywhere in the human body. Forty-three per cent occur in the extremities with two-thirds of them in the lower limb and one-third in the upper limb. Thirty-four per cent of sarcomas are intra-abdominal consisting of 19% visceral and 15% retroperitoneal lesions [2]. Primary pure sarcomas are very rare and constitute 0.2–1.0% of all breast malignancies [3].
Several histological subtypes of breast sarcomas have been described as case series or case reports due to the multiple different cells present in the mesenchymal tissue of the mammary gland, such as endothelial, muscle and fat cells. The most common subtypes are malignant fibrohistiocytoma, fibrosarcoma, angiosarcoma and spindle cell sarcoma. Less common subtypes are liposarcoma, leiomyosarcoma, rhabdomyosarcoma, osteosarcoma, synovial sarcoma, neurosarcoma, stromal sarcoma, chondrosarcoma and hemangiopericytoma. Angiosarcomas appear to be the commonest histological subtype in radiation-induced sarcomas of the breast [4, 5]. The majority of breast sarcomas present without a causative factor. The main risk factor for the development of breast sarcomas is previous radiation treatment for breast carcinoma and non-Hodgkin's lymphoma. Other recognized factors are chronic lymphoedema, exposure to vinyl chloride and artificial implants [6] as well as Li-Fraumeni syndrome through a p53 mutation [1].
CASE REPORT
A 45-year-old healthy Caucasian female patient presented in the emergency department with sudden onset of left breast erythema. On examination, she had an oedematous, tender, erythematous left breast with areas of blistering, epidermolysis and necrosis. Observations demonstrated systemic inflammatory response syndrome with tachycardia, pyrexia and hypotension. Investigations demonstrated raised inflammatory markers, electrolyte abnormalities and severe anaemia. An ultrasound scan revealed the presence of gas and fluid-filled pockets.
The Laboratory Risk Indicator for Necrotising Fasciitis score can be utilized to risk stratify patients presenting with signs of cellulitis to determine the likelihood of necrotizing fasciitis. A score >6 of 13 indicates that necrotizing fasciitis should be seriously considered. In this case, a score of 8 was allocated for serum sodium of 126 mmol/l, haemoglobin of 6.6 g/dl and C-reactive protein of 306.7 mg/l. Her other biochemical markers were normal, including serum creatinine of 68 µmol/l, plasma glucose of 8.8 mmol/l and a leukocyte count of 11.5 × 109/l. A provisional diagnosis of necrotizing fasciitis was made, and the patient was referred to the breast surgeons. She was taken to the theatre immediately, where she underwent a mastectomy.
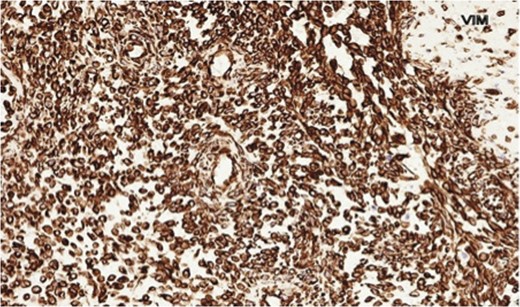
The histopathology demonstrated that the breast was partially replaced by a partly cystic and partly solid necrotic lesion. The cystic cavity contained blood clots. A microscopic examination showed an atypical undifferentiated spindle cell lesion with a fascicular architecture, pleomorphism and marked mitotic activity with areas of ulceration and necrosis (Figs 1 and 2). The initial set of immunostains performed showed that tumour cells were negative for cytokeratins (Fig. 3), S100 and LCA. The tumour cells showed diffuse and strong vimentin positivity (Fig. 4). Further immunocytochemical labelling showed weak expression of CD99, patchy strong expression of CD10 and patchy weak expression of smooth muscle actin. Cytokeratins (CAM 5.2) and EMA were expressed and there was no tumour-specific expression of CD34, desmin or myogenin. Fluorescent In-situ hybridization analysis failed to demonstrate evidence of a t(x:18) translocation using the LYS-SYT break apart probe. The diagnosis of a Grade 3 undifferentiated spindle cell sarcoma was made and although the tumour was reported to have a clear resection margin (5 mm) in most areas, the presence of necrosis and granulation tissue reaching the deep margin made the status of the margin uncertain.
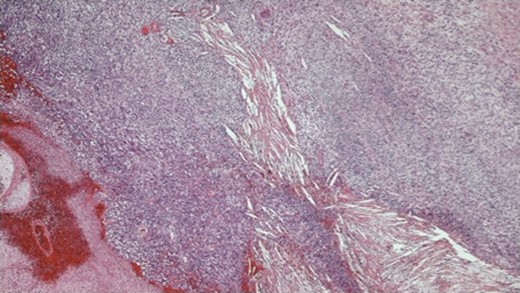
Photomicrograph ×4 H&E; spindle cell tumour with areas of haemorrhage and necrosis.
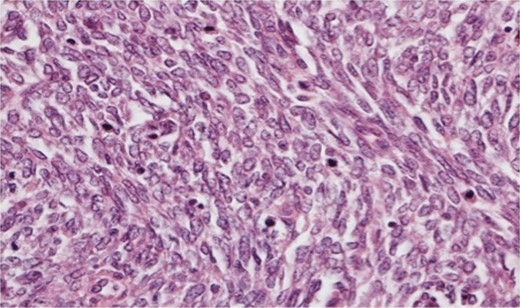
Photomicrograph ×40 H&E; pleomorphic spindle cells in fascicles with several mitoses.
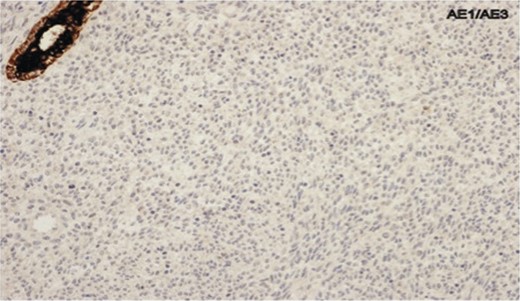
Photomicrograph showing negative cytokeratin AE1/AE3 staining in spindle cells.
Staging computed tomography scan demonstrated left axillary lymphadenopathy and a small left-sided basal effusion. Following discussion of the case at the multidisciplinary meeting, radical completion mastectomy was recommended. In addition, she underwent adjuvant radiotherapy. Reconstruction was with a split thickness skin graft by the plastic surgery team prior to her radiotherapy treatment (Fig. 5).
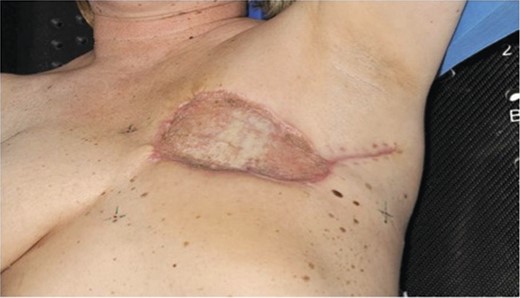
Postoperative photograph demonstrating split thickness skin graft reconstruction.
DISCUSSION
Necrotizing fasciitis is a highly lethal infection that causes rapidly spreading necrosis of fascia and subcutaneous tissues, sometimes involving muscle and skin. Recent reports have reviewed the clinical features, highlighting the potential pitfalls in diagnosis [7]. Treatment is with haemodynamic support of sepsis, broad spectrum antibiotics, intravenous immunoglobulin and extensive debridement. Following debridement in this patient, sections from the specimen demonstrated an undifferentiated spindle cell sarcoma with areas of haemorrhage and necrosis, contrary to the initial presentation of presumed necrotizing fasciitis. This resulted in a significant alteration in her management plan.
The treatment for breast sarcomas is planned by a multidisciplinary team and follows the treatment model of sarcomas in other locations. Surgery is the primary treatment. Localized tumours should be treated by complete excision, while mastectomy should be performed for more sizeable tumours. Adequate surgical excision seems to be one of the most important prognostic factors along with the tumour's diameter and grade. Axillary dissection is unnecessary for breast sarcomas, as they rarely metastasize via the lymphatic system [8]. Adjuvant and neoadjuvant chemotherapy and radiotherapy should be considered in high-risk cases. While chemotherapy is the mainstay of treatment for advanced systemic disease, radiotherapy has a role in lymphatic metastasis and preventing loco-regional recurrence. The reported 5-year survival in patients with breast sarcomas varies between 40 and 91% in different studies [9,10].
REFERENCES
- radiation therapy
- epithelium
- heterogeneity
- decision making
- necrotizing fasciitis
- lymphatic metastasis
- lymphoma, non-hodgkin
- middle-aged adult
- patient care team
- sarcoma
- breast
- neoplasms
- breast cancer
- breast conserving surgery
- breast sarcoma
- operative management of breast cancer
- interdisciplinary treatment approach
- spindle-cell sarcoma



