-
PDF
- Split View
-
Views
-
Cite
Cite
Yu-Ting Wu, Hua-Koon Wu, Shou-Tung Chen, Chih-Jung Chen, Dar-Ren Chen, Hung-Wen Lai, Fibroadenoma progress to ductal carcinoma in situ, infiltrating ductal carcinoma and lymph node metastasis? Report an unusual case, Journal of Surgical Case Reports, Volume 2017, Issue 5, May 2017, rjx064, https://doi.org/10.1093/jscr/rjx064
Close - Share Icon Share
Abstract
Fibroadenoma of the breast is the most common benign neoplasm in young women who present with a palpable, movable mass. Malignancy inside fibroadenomas is rare, with reported rates ranging from 0.002% to 0.125%. Carcinoma in situ inside a fibroadenoma is usually found incidentally when tumours are excised. Whether fibroadenoma is a risk factor for breast cancer remains controversial. Due to the rarity of carcinomas inside fibroadenomas, medical institutes have little experience with this phenomenon. We report an unusual case in which progression occurred from benign fibroadenoma to ductal carcinoma in situ, infiltrating ductal carcinoma and lymph node metastasis. A nipple-areolar complex-preserving mastectomy with immediate breast reconstruction with a gel implant and contralateral augmentation was performed. No local recurrence or metastasis was found during 5 years of follow-up.
INTRODUCTION
Most breast lumps in young female patients are found to be fibroadenomas [1–3], which are usually benign and self-limiting. Asymptomatic fibroadenomas can be conservatively managed [2]. Excision may be needed if the mass enlarges or if other atypical symptoms occur. Cancer within fibroadenoma is usually found incidentally during pathologic examination, with reported incidences ranging from 0.002% to 0.125% [4–6].
Malignant findings inside fibroadenomas are usually carcinoma in situ; <15% are invasive breast cancers [7]. Whether fibroadenoma is a risk factor for breast cancer remains controversial [5, 8–10]. Here, we present the case report of a patient with a long history of fibroadenoma that then progressed during follow-up to ductal carcinoma in situ (DCIS), invasive ductal carcinoma (IDC) and lymph node metastasis. The treatment details are reported along with a literature review.
CASE REPORT
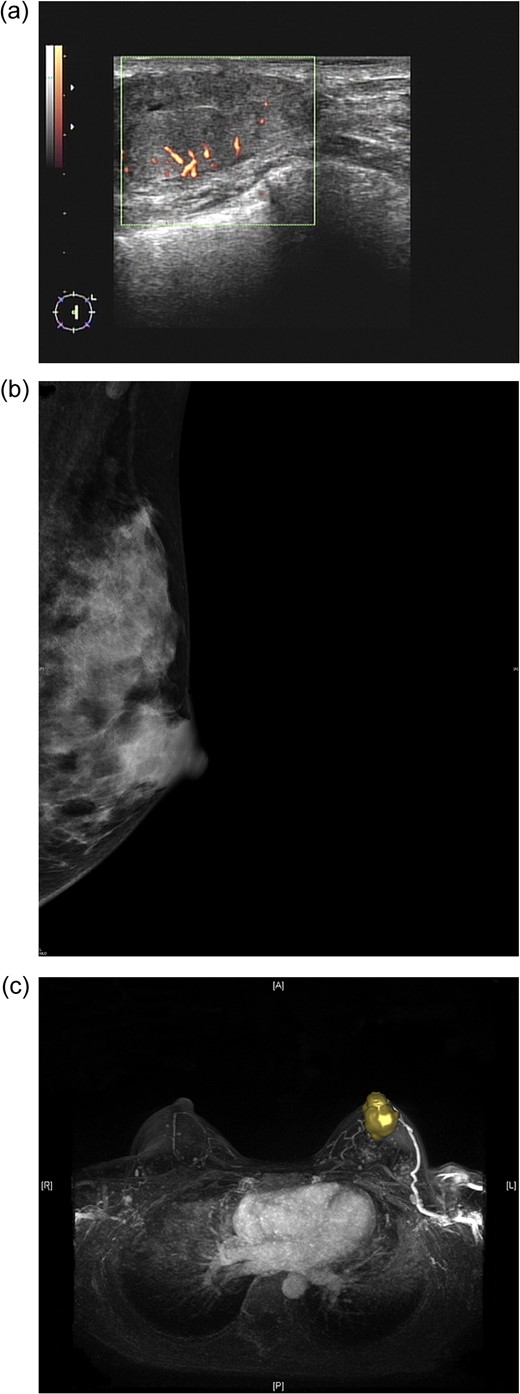
(a) Sonogram of the left breast reveals a 3.5-cm hypoechoic lesion with increased vascularity in the 2 o'clock/1-cm region. (b) Mammogram (mediolateral-oblique view) of the left breast shows a well-defined sharply circumscribed mass. (c) A maximal intensity projection magnetic resonance imaging image of the left breast revealed a 4.0-cm well-defined tumour with an engorged drainage vein beneath the nipple areolar complex region.
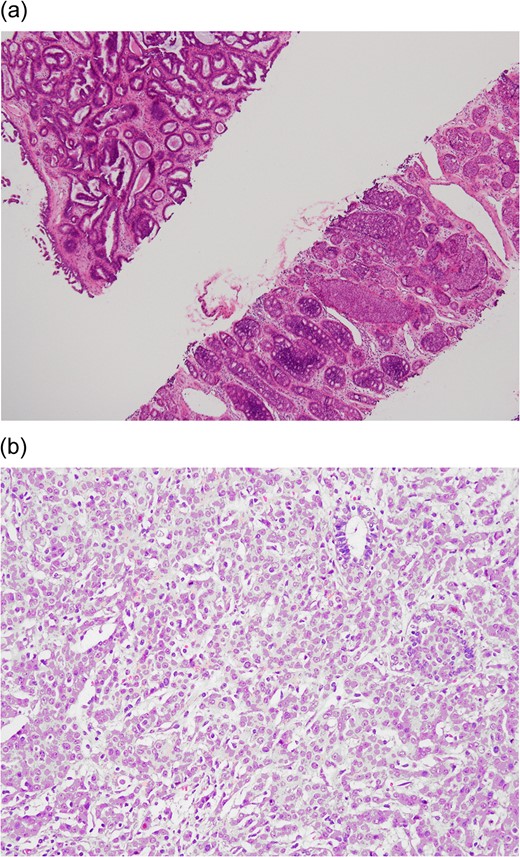
(a) Preoperative CNB (low-power field). The upper left tissue shows a complex glandular growth pattern in the fibro-myxomatous stroma. The lower right tissue shows DCIS in solid and cribriform patterns. (b) Postoperative final pathology (high power field). Grade II infiltrating ductal carcinoma characterized by nesting to glandular structure with intermediate-sized nuclei and rare mitotic figures.
Preoperative mammography revealed one well-defined, sharply circumscribed lesion (Fig. 1b). Magnetic resonance imaging (MRI) showed one 4-cm circumscribed mass beneath the nipple-areolar complex (NAC) region of the left breast, with focal haemorrhage, nipple retraction, skin thickening, dilated ducts and lymphadenopathy (Fig. 1c).
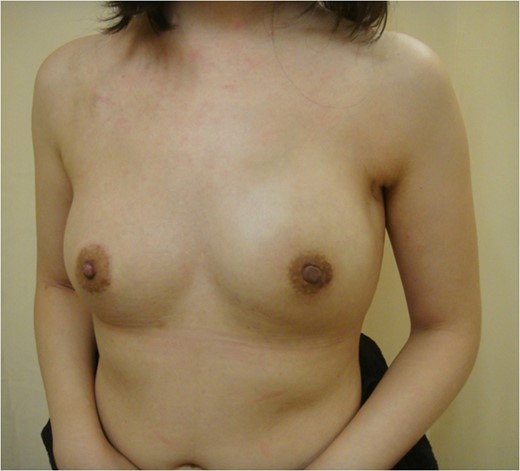
Postoperative view 3 months after nipple-sparing mastectomy with immediate breast reconstruction with a gel implant combined with contralateral augmentation.
The postoperative pathology report revealed IDC with DCIS arising from a complex background of fibroadenoma (Fig. 2b). The stage classification was pT1a (0.5 cm) N1(1/18)M0, Stage IIA. The hormone receptor status was oestrogen receptor–positive and progesterone receptor–positive. The HER-2 receptor was not overexpressed, and the Ki-67 level was 10%. In consideration of the patient's young age (31 years) and lymph node metastasis, she underwent adjuvant chemotherapy with 5-fluorouracil (500 mg/m2), epirubicin (90 mg/m2) and cyclophosphamide (500 mg/m2) (four cycles) followed by docetaxel (75 mg/m2) (four cycles). She is currently undergoing adjuvant endocrine therapy with tamoxifen (20 mg/per day) and regular follow-up at our outpatient clinic. There has been no disease recurrence or distant metastasis.
DISCUSSION
We present the rare case of a patient with a diagnosis of CNB with DCIS arising from fibroadenoma. When the tumour was excised, IDC with lymph node metastasis was found. This unusual case demonstrates the rare transition of benign fibroadenoma to malignant DCIS, invasive carcinoma and then lymph node metastasis.
Whether fibroadenoma is a risk factor for breast cancer remains uncertain, and several reports have discussed this phenomenon [5, 10–13]. Dupont et al. [5] reported that the risk of invasive breast cancer was 2.17 times higher in patients with fibroadenoma and increased to 3.10 times higher in complex fibroadenoma. The risk of malignant change persisted even 20 years after the diagnosis of fibroadenoma. Other studies also provide strong evidence that fibroadenoma with hyperplasia is associated with an increased risk of breast cancer [11, 12]. However, Bonardi et al. [10] reported that the observed transformation of fibroadenoma to breast cancer was due to selection bias. Levi et al. [13] claimed that the evidence was too weak to support the connection of fibroadenoma to subsequent breast cancer.
Imaging studies may suggest characteristics of carcinomas arising from fibroadenomas [14–16]. In sonographic examination of fibroadenomas, irregular shape and contour, extensive hypo-echogenicity, shadowing, echogenic halo and distortion of surrounding tissue are considered to be hints of suspicious malignancy [15]. On mammography, irregular shape, ill-defined spiculated margins or pleomorphic/linear micro-calcifications should also be considered signs of malignancy [14]. With dynamic MRI, benign fibroadenoma and DCIS/IDC can be differentiated according to differences in vascularity [16]. Parameter colour maps can also demonstrate the extent of DCIS within a fibroadenoma [16]. However, in the case described here, sonography, mammography and MRI revealed a well-defined, sharply circumscribed hypoechoic lesion with increased vascularity (Fig. 1a–c). Preoperative imaging to differentiate benign from malignant fibroadenoma was not easy, and CNB was therefore performed to obtain a tissue diagnosis.
The surgical treatment of benign fibroadenoma is to excise the mass. Tumourectomy or lumpectomy may be sufficient if the surgical margin is free of cancer or if only lobular carcinoma in situ is found inside the fibroadenoma [17, 18]. However, if the resection margin is involved or close, further wide excision may be needed [5]. In the current case, because the tumour was large in a relatively small breast, a nipple-sparing mastectomy was performed instead of breast conservative surgery. Due to the usually well-defined capsule-like nature of fibroadenomas [3, 19], malignancy inside a fibroadenoma is often regarded as more benign than other types of breast cancer. Intraoperative sub-nipple biopsy was performed to decide whether the NAC could be preserved [20].
This unusual case involved the transition of benign fibroadenoma to malignant DCIS, IDC and lymph node metastasis. Although rare, the risk of malignancy inside a fibroadenoma should be kept in mind. Adequate action (CNB or excision) should be performed when an enlarging mass or atypical presentation of fibroadenoma is found.
FUNDING
This study was supported by Changhua Christian Hospital Breast Cancer Research Program (105-CCH-IRP-025).
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
The authors would like to thank Ya-Ling Lin for her assistance in this study.
REFERENCES
Author notes
Dr Yu-Ting Wu and Hua-Koon Wu contribute equally in this paper.



