-
PDF
- Split View
-
Views
-
Cite
Cite
Khortnal Delvecchio, Susan Seman, Successful surgical excision of a massive symptomatic partially obstructing Brunner's gland hamartoma: a case report, Journal of Surgical Case Reports, Volume 2016, Issue 12, 1 December 2016, rjw206, https://doi.org/10.1093/jscr/rjw206
Close - Share Icon Share
Abstract
Brunner gland function within the duodenal epithelium is secretion of alkaline mucin to counteract acidic chyme. These glands may grow beyond the duodenal wall to become hamartomas. Rarely, they become large enough to cause obstructive symptoms in the upper gastrointestinal tract. We report a case of one of the largest lesions identified in the literature causing obstruction symptoms. A previously asymptomatic 65-year-old gentleman presented to the emergency department with a single-day history of colicky abdominal pain, cramping and vomiting. After initial imaging with computed tomography was inconclusive, a subsequent esophagogastroduodenoscopy with biopsy and small bowel series was performed indicating a pedunculated polypoid mass. Our patient underwent an uncomplicated exploratory laparotomy with duodenotomy and Heineke-Mikulicz pyloroplasty for resection of a massive Brunner's gland hamartoma. For similar presentations, we recommend this technique.
INTRODUCTION
Initially described by Johann Brunner in 1688, the eponymous Brunner glands are branched acinotubular structures specific to the duodenal submucosa [1]. They secrete an alkaline fluid of mucin as well as pepsinogen and urogastrone protecting the epithelium from and inhibiting secretion of gastric chyme, respectively [1–3]. Rarely, these glands grow beyond the duodenal wall, becoming hamartomas, leading to obstruction, ulceration or intussusception [4]. The majority, however, are asymptomatic and incidentally discovered, thus do not require treatment [4]. In addition, a significant relationship has been established with these lesions and Helicobacter pylori in the literature, and consequently one prevailing hypothesis to their development reflects an inflammatory response from associated hyperchlorhydria [1, 5].
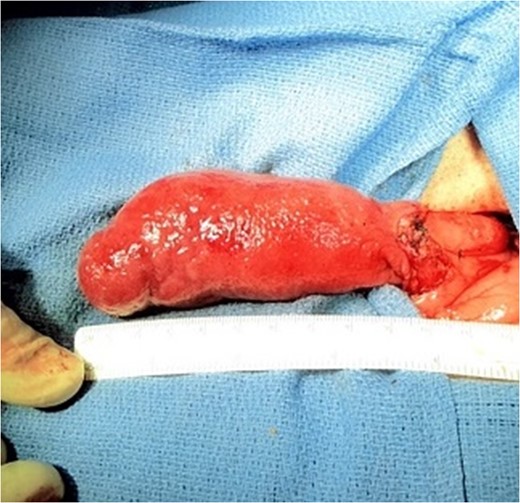
Brunner's gland hamartomas are relatively rare, found in <1 in 10 000 individuals and roughly 5% of all duodenal tumors [6]. They are most prevalent in the fifth and sixth decades and represent the genders and races equally [2, 3, 5]. Seventy percent are located in the duodenal bulb, and they become smaller and fewer distally [2, 6]. Due to their average size of 1–2 cm, the majority are asymptomatic and thus discovered on endoscopy or imaging; however, when over 2 cm they may obstruct [2, 3, 7, 8]. Since first reported, around 200 cases have been reported in the literature with only a handful measuring over 5 cm, the largest being 12 × 10 × 8 cm [7, 9]. The vast majority of these lesions are benign, with few reports citing malignant features, thus resection should be performed for definitive diagnosis [2, 10].
CASE REPORT
A 65-year-old African-American male presented to the emergency department with a 1-day history of 10/10 colicky, generalized upper abdominal pain and cramping that forced him into the fetal position after eating sausage and eggs. He admitted to nausea with dry-heaving and denied changes in bowel function or any alleviating factors. His examination consisted of stable vital signs and was devoid of abdominal pain or tenderness.
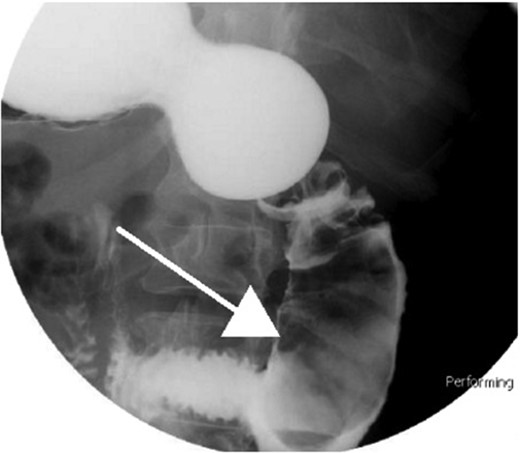
Small bowel series showing duodenal filling defect (posterior view).
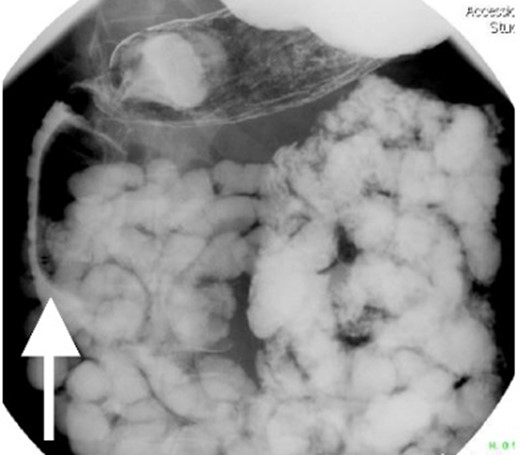
Small bowel series showing duodenal filling defect (anterior view).
On postoperative day (POD) 1, the patient had one episode of emesis after premature self removal of nasogastric tube (NGT) and after reinsertion there were no other significant complications. The patient passed flatus on POD 3 and reported bowel movements on POD 4 coinciding with NGT removal. He tolerated diet advances until discharge on POD 6.
DISCUSSION
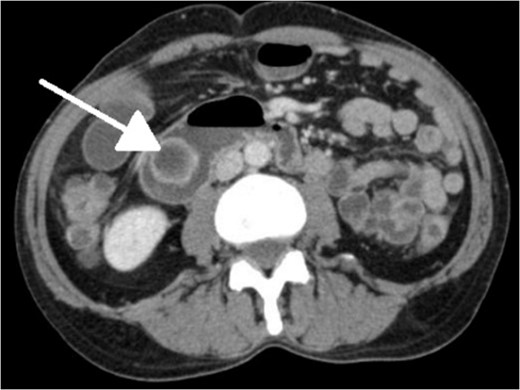
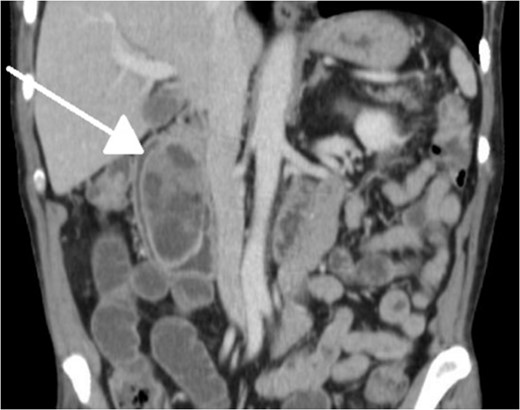
Limitations to our workup include failing to test for H. pylori or excessive gastric acid secretion, which has been linked to over 70% of lesions [1, 9]. Although treating H. pylori has not demonstrated lesion regression or recurrence in the literature, anecdotal evidence points toward this logic [1]. In addition, although our patient's CT scan did indicate dilatation of the pancreatic duct as well as the CBD, we did not draw bilirubins, which could have pointed toward biliary obstruction, recurrent pancreatitis and/or biliary fistula that occasionally manifests [3, 7, 9].
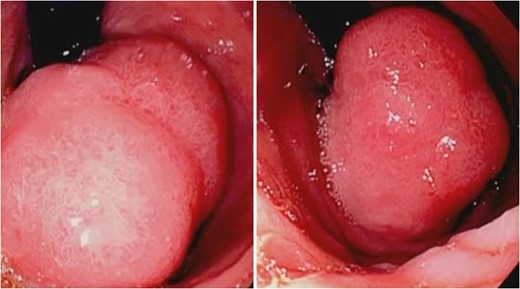
With regard to diagnostic workup, the literature has described cases with similar results to ours. Small bowel contrast studies frequently show smooth-walled polypoid filling defects within the duodenal bulb, and CT imaging is useful for delineation of adjacent structures including the common duct and pancreas [1]. Moreover, similar to the pathological results found in our patient, upper endoscopy biopsies are often equivocal as they are submucosal-based lesions [1]. The most useful diagnostic study, however, is endoscopic ultrasound, which may help distinguish the consistency and bowel layer of origin [2, 6], which unfortunately was not available at our institution.
Massive Brunner's gland hamartomas have rarely been described in the literature and even rarer are they diagnosed after only a single day of symptoms. Moreover, our case of a ‘giant’ hamartoma is significant as one of only a few larger than 5 cm ever described. Considering typical lesions tend to be smaller, endoscopic surgical excision is preferred for reduced morbidity and length of stay. In fact, recent literature has described successful snare cautery of lesions up to 5 cm, or via piecemeal if too difficult [1, 3, 4]. Laparotomy with duodenectomy, however, is preferred over endoscopic methods for massive lesions, those with refractory bleeding, complete obstruction or if there is concern for malignancy (9). This leads to the suggestion of possible future randomized studies featuring outcomes of endoscopic versus open surgical excision of these masses.
Conflict of interest statement
None declared.
REFERENCES
- computed tomography
- acute abdominal pain
- biopsy
- epithelium
- upper gastrointestinal endoscopy
- hamartoma
- bodily secretions
- brunner glands
- emergency service, hospital
- mucins
- vomiting
- diagnostic imaging
- duodenum
- duodenotomy
- laparotomy, exploratory
- heineke-mikulicz pyloroplasty
- excision
- upper gastrointestinal tract
- small bowel radiography



