-
PDF
- Split View
-
Views
-
Cite
Cite
Hounaida Mahfoud, Ibtissam Bensrhir, Meryem Abbouch, Chourouq Mustapha Eid, Mariame Meziane, Samia Sassi, Najat Lamalmi, Najia Zeraidi, Aziz Baidada, Massive cellular angiofibroma of the vulva: a case report, Journal of Surgical Case Reports, Volume 2024, Issue 8, August 2024, rjae508, https://doi.org/10.1093/jscr/rjae508
Close - Share Icon Share
Abstract
Cellular angiofibroma is a rare benign mesenchymal tumor, typically occurring in the vulvar region of middle-aged women. This report highlights the importance of histological analysis in diagnosing this uncommon condition and emphasizes its benign nature and straightforward management. We present a case of a 58-year-old North African woman who had a large, well-defined mass in the left labia majora, which had been evolving over 2 years. MRI confirmed the resectability of the tumor by delineating its boundaries. The tumor, despite its slow growth leading to delayed diagnosis, was effectively treated with wide surgical excision. Diagnosis was confirmed through histological and immunohistochemical evaluations, revealing spindle cell proliferation with thick-walled vessels. Cellular angiofibroma, although initially alarming due to its size, is generally managed successfully with surgery and prognosis is favorable with a low risk of recurrence.
Introduction
Cellular angiofibroma (CA) is an uncommon benign tumor typically observed in middle-aged women, with its initial description dating back to 1997 [1]. Along with myofibroblastoma and angiomyofibroblastoma, it fits within a histologic spectrum of mesenchymal neoplasms, characterized by spindle cell and vascular proliferation [2]. It is the least frequent among the three entities and generally appears in the lower female genital tract, especially the vulva [3]. While it usually presents as a small, well-circumscribed mass, its slow growth may lead to delayed diagnosis, resulting in sizable lesions by the time of diagnosis [4]. Consequently, treatment through wide surgical excision with clear margins can be challenging, highlighting the importance of early consultation for any soft masses in the vulva.
Herein, we present a case of a massive vulvar CA in a 58-year-old woman, successfully managed through complete surgical excision. We aim to outline, through this report, the diagnostic process, histological features, and treatment approach.
Case presentation
A 58-year-old North African menopausal woman, with no significant medical or surgical history, was referred by a dermatologist to our department for management of a vulvar mass. She had no history of oral contraceptive use, genital warts or family history of gynecological cancer. She had noticed a small growth on her left labia that has increased progressively in size over the previous 2 years, causing discomfort while walking. It was otherwise painless.
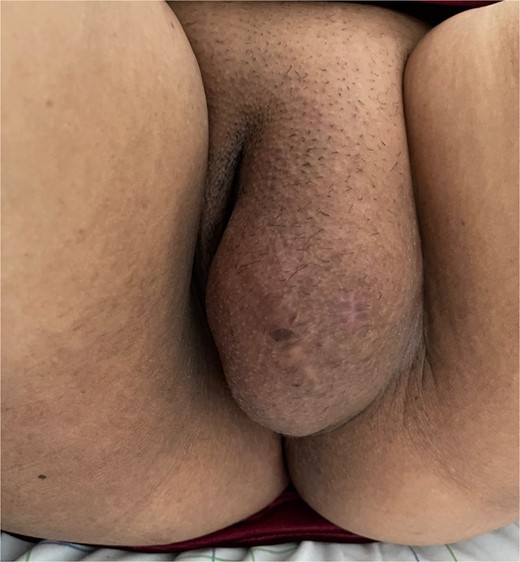
Upon physical examination, a well-defined mass spanning the left labia majora, measuring 10 cm in length, was noted. The mass was firm, non-tender, and mobile in the superficial plane but adherent to the deep plane (Fig. 1). The overlaying skin showed no inflammatory signs. There were no palpable lymph nodes, and pelvic examination was unremarkable.
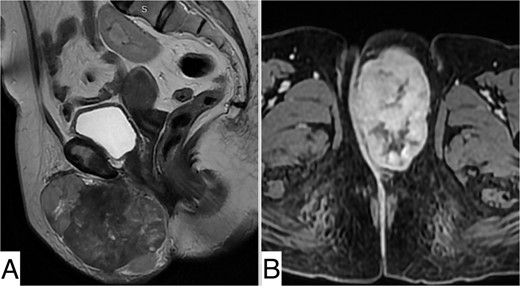
Pelvic MRI revealed a well-defined, rounded mass centered on the left labia majora, isointense on T1 compared with muscle, heterogeneously enhanced after Gadolinium injection, indicating necrotic areas, measuring 98 × 57 × 77 mm. It was in close contact with the left ischiopubic ramus and infiltrated the lower two-thirds of the vesical urethra without invading it. It extended into the retro-pubic fat more markedly on the left. The right labium majus, clitoris, and adjacent perineal structures were preserved. The vagina, cervix, anal canal, and rectum were unaffected (Fig. 2).
Surgical resection for diagnostic and therapeutic purposes was scheduled. The mass was resected en bloc via an elliptical skin incision under Foley catheterization.
Upon macroscopic examination, the mass measured 120 × 80 × 50 mm and was well-defined, whitish, fasciculated with a firm consistency. Histopathological examination showed a mesenchymal proliferation consisting of regular, monomorphic fibroblastic cells amidst a background of capillary vessels with thickened walls. No mitoses or areas of necrosis were observed. All margins were clear. Immunohistochemical analysis showed positive staining of tumor cells with anti-CD34 antibodies, while staining with anti-protein S100, anti-desmin, and anti-smooth muscle actin (SMA) antibodies was negative. Thus, the diagnosis of CA was confirmed (Fig. 3).
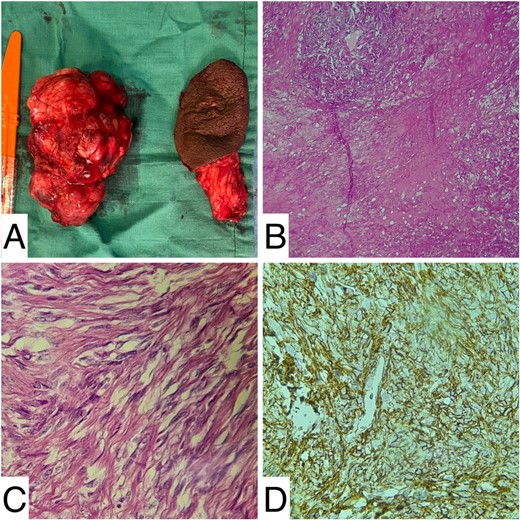
Histology images: (A) gross image of the resected mass; (B and C) medium and high magnification showing fibroblasts and capillary vessels; (D) CD34 positivity at immunochemistry.
The postoperative course was uneventful. The patient was discharged after 3 days and was closely monitored during follow-up to assess for any signs of wound infection, particularly due to its location in the perineal area. There were no signs of recurrence after 1 year.
Discussion
CA is a rare benign mesenchymal tumor, first described by Nucci et al. in 1997 [1]. It is found in the genitourinary tract of both sexes, predominantly in the vulvovaginal region in women and the inguinoscrotal region in men [5]. It typically appears around menopause in women, around the age of 50 years [6].
Clinically, CA is generally asymptomatic presenting as a small, painless mass that grows slowly within the superficial soft tissue, often leading to delayed consultation, as was the case with our patient who sought medical attention a year after the tumor appeared [4]. It is well-circumscribed with a smooth surface and an average size of 3 cm, but it can reach a substantial size if untreated, up to 30 cm as described by Reder et al. [7]. Our patient’s case is among the largest described in the literature.
The differential diagnosis of CA includes Bartholin’s cyst and crural hernia, which can be easily ruled out by physical examination and ultrasound [8]. Indeed, the latter shows CA as a well-marginated solid mass with hypoechoic or heterogeneous architecture. However, it is MRI that provides a comprehensive assessment of the tumor, offering detailed information about its location, size, and its relationship to adjacent organs and soft tissue structures. CA appears then as a well-circumscribed variably heterogeneous lesion, avidly enhanced after Gaolinium injection [9]. It is usually encapsulated [10].
Nonetheless, CA is primarily a histological diagnosis. It falls under the category of mesenchymal tumors of the vulva, which includes myofibroblastoma, angiomyofibroblastoma, and aggressive angiomyxoma [2, 10]. These histological entities are all comprised of bland spindled cells, a prominent vascular component, and adipose tissue [8]. Grossly, CA is a round or oval often encapsulated well-circumscribed tumor, white or yellow in color but tan or gray on cross-section, with a soft to rubbery consistency [10, 11].
Microscopically, it features two main components: spindle cell proliferation and vascular proliferation. The spindle cells are short, uniform arranged randomly or in a fascicular pattern, surrounded by a stroma of fine collagen fibers and clusters of mature adipocytes. The vascular proliferation includes small to medium-sized vessels with mural hyalinization [10, 11].
Immunochemistry staining is often positive for CD34 (60%) but negative for SMA and desmine, as seen in our case [2]. Estrogen and progesterone receptors are present as well, however with no diagnostic significance, given the common expression of hormone receptors in genital mesenchymal lesions [4].
Genetically, CA typically exhibits the monoallelic deletion of RB1 and FOXO1 on chromosome 13q14, a chromosomic abnomality shared with myofibroblastoma [12].
Treatment is essentially surgical, comprising in a wide excision with clear margins. Direct access is usually the approach for vulvar lesions, however, transperineal, transabdominal, and laparoscopic access can be used depending on the location. The encapsulated nature of the lesion generally makes surgical removal straightforward with minimal blood loss [10, 13]. For large tumors, complications such as vulvar hematoma, vesicovaginal and rectovaginal fistulas, and skin closure tension may occur. To mitigate these risks, a surgical drain may be used, reconstructive surgery may be necessary, and a multidisciplinary approach involving both colorectal and gynecological surgeons may be required [10, 14].
Non-surgical treatments include hormonal therapy and a combination of high-frequency electric therapy with 5-ALA photodynamic therapy. These are primarily used for small lesions and have shown variable outcomes. Their efficacy in treating large CAs remains to be proven [5, 14].
In terms of prognosis, CA is a benign tumor with a low recurrence rate, and no metastases have been reported [9, 11].
Author contributions
All authors read and approved the final manuscript.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
Not applicable.
Consent
A consent was obtained from the patient to publish this case report and accompanying images. On request, a copy of the written consent is available for review by the Editor-in-Chief of this journal.



