-
PDF
- Split View
-
Views
-
Cite
Cite
Ryosuke Mizukami, Akiko Nakazawa, Takahiro Einama, Risa Kariya, Mayuko Ohara, Koki Ichio, Fukumi Konno, Kazuki Kobayashi, Naoto Yonamine, Ibuki Fujinuma, Takazumi Tsunenari, Yasuhiro Takihata, Mikiya Takao, Eiji Shinto, Hideki Ueno, Yoji Kishi, Ruptured large hepatic cyst with elevated serum and ascites CA19-9 level, Journal of Surgical Case Reports, Volume 2024, Issue 8, August 2024, rjae309, https://doi.org/10.1093/jscr/rjae309
Close - Share Icon Share
Abstract
Tumor markers such as carbohydrate antigen 19-9 (CA19-9) are generally useful in ruling out malignancy of hepatic cysts. The patient was a 72-year-old man who had a ruptured liver cyst in the right liver, which had been noted since he was 67 years old at another hospital. The initial laboratory tests demonstrated elevated CA19-9 (193 784.3 U/mL). We made the diagnosis with a simple ruptured liver cyst from magnetic resonance imaging and cytological examination of ascites, and laparoscopic fenestration with drainage of the abdominal fluid was performed. Pathological diagnosis of the resected wall cyst was non-parasitic simple hepatic cyst with acute inflammation and hemorrhage. The patient’s serum levels of CA19-9 were 164.0 U/mL on postoperative day 23. The follow-up abdominal computed tomography scan performed 2 months later did not any finding of tumor.
Introduction
Simple hepatic cyst is a benign disease in which follow-up is often selected when asymptomatic. If the patient has the symptoms such as abdominal pain, we need to consider invasive treatments. In such a situation, we often have difficulty in distinguishing benign hepatic cysts from malignant lesions especially when solid nodules are not evident by imaging studies. Elevated tumor markers, such as CA19-9 in the serum, are usually considered to suggest the diagnosis of malignancy, but their diagnostic accuracy has not well been evaluated. We report a case of a ruptured large hepatic cyst with elevated serum CA19-9 levels but the pathologic evaluation of the cyst wall resected by laparoscopic fenestration showed no findings to suggest malignancy.
Case report
A 72-year-old was referred to our department with the diagnosis of ruptured liver cyst with bloody ascites by abdominal contrast-enhanced computed tomography (CT). The patient had been admitted in the orthopedic department in another hospital following the surgery for his ankle fracture and sudden right upper abdominal pain. He had past history of hypertension, atrial fibrillation and neck surgery for cervical myelopathy in his 50s. A large liver cyst had been pointed out since his 60s.
On referral to our hospital, his vital signs were within normal limits. The abdominal examination revealed rebound tenderness in his right upper quadrant region. There was no abdominal distension. The initial laboratory tests demonstrated a normal hemoglobin (10.2 g/dl) and elevated C-reactive protein levels (9.6 mg/dl). The results of serum tumor markers were as the follows: CA19-9, 193 784.3 U/ml; carbohydrate antigen 125 (CA125), 91.6 U/ml; carcinoembryonic antigen (CEA), 2.1 ng/ml; α-fetoprotein (AFP), 3.0 ng/ml; des-γ-carboxy prothrombin (DCP), 19 mAU/ml (Table 1). Dynamic CT showed a large cystic lesion (17 cm × 14 cm × 12 cm) in the right liver and discontinuation of the cystic wall and retention of ascites mainly the right subphrenic region (Fig. 1). T2-weighted single shot turbo spin echo magnetic resonance imaging (MRI) showed a-low intensity region with dorsal predominance in the high-intensity area and fluid–fluid level (Fig. 2). Gadoxetic acid (Gd-EOB) enhanced MRI didn’t show any lesion suggesting mural nodule in the cystic wall in any phase (Fig. 3). Paracentesis showed bloody fluid and the tumor marker levels in the sampled ascites were as the follows: CA19-9, 2 672 400.0 U/ml; CA125, 2618.9 U/ml; CEA, 41.7 ng/ml; AFP, 1.4 ng/ml; and DCP, 10 mAU/ml (Table 1). Cytology of the ascites showed no findings of malignancy.
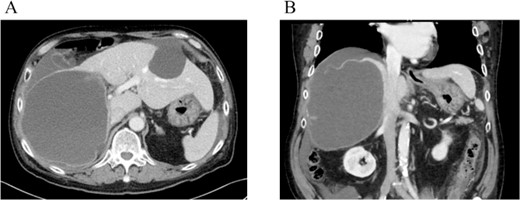
Dynamic CT showed a large cystic lesion (17 cm × 14 cm × 12 cm) in right liver (A), and also the cystic wall rupture under the right diaphragm (B).
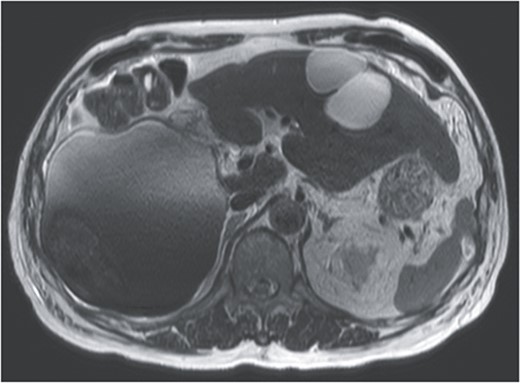
T2-weighted single shot turbo spin echo MRI showed a low intensity region with dorsal predominance in the high-intensity area, and fluid–fluid level.
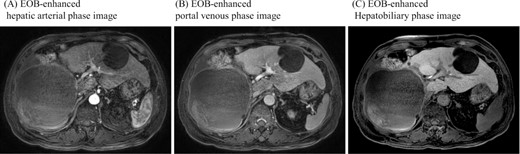
Gadoxetic acid (EOB) enhanced MRI didn’t show contrast effect with a cystic lesion in any phase.
| Complete blood count . | . | Biochemistry . | . | Abdominal fluid . | . |
|---|---|---|---|---|---|
| WBC | 4600/μL | T-bil | 0.81 mg/dL | Color | Dark red |
| Hb | 10.2 g/dL | AST | 18 U/L | CA19-9 | 2 672 400 U/mL |
| Plt | 33.9 × 104/μL | ALT | 20 U/L | CA125 | 2618.9 U/mL |
| ALP | 74 U/L | CEA | 41.7 ng/mL | ||
| γ-GTP | 18 U/L | AFP | 1.4 ng/mL | ||
| Cr | 0.79 mg/dL | PIVKA-II | 10 mAU/mL | ||
| CRP | 9.6 mg/dL | T-bil | 5.25 mg/dL | ||
| CA19-9 | 193 784.3 U/mL | Amylase | 11 U/L | ||
| CA125 | 91.6 U/mL | ||||
| CEA | 2.1 ng/mL | Cytology | class II | ||
| AFP | 3.0 ng/mL | ||||
| PIVKA-II | 19 mAU/mL |
| Complete blood count . | . | Biochemistry . | . | Abdominal fluid . | . |
|---|---|---|---|---|---|
| WBC | 4600/μL | T-bil | 0.81 mg/dL | Color | Dark red |
| Hb | 10.2 g/dL | AST | 18 U/L | CA19-9 | 2 672 400 U/mL |
| Plt | 33.9 × 104/μL | ALT | 20 U/L | CA125 | 2618.9 U/mL |
| ALP | 74 U/L | CEA | 41.7 ng/mL | ||
| γ-GTP | 18 U/L | AFP | 1.4 ng/mL | ||
| Cr | 0.79 mg/dL | PIVKA-II | 10 mAU/mL | ||
| CRP | 9.6 mg/dL | T-bil | 5.25 mg/dL | ||
| CA19-9 | 193 784.3 U/mL | Amylase | 11 U/L | ||
| CA125 | 91.6 U/mL | ||||
| CEA | 2.1 ng/mL | Cytology | class II | ||
| AFP | 3.0 ng/mL | ||||
| PIVKA-II | 19 mAU/mL |
| Complete blood count . | . | Biochemistry . | . | Abdominal fluid . | . |
|---|---|---|---|---|---|
| WBC | 4600/μL | T-bil | 0.81 mg/dL | Color | Dark red |
| Hb | 10.2 g/dL | AST | 18 U/L | CA19-9 | 2 672 400 U/mL |
| Plt | 33.9 × 104/μL | ALT | 20 U/L | CA125 | 2618.9 U/mL |
| ALP | 74 U/L | CEA | 41.7 ng/mL | ||
| γ-GTP | 18 U/L | AFP | 1.4 ng/mL | ||
| Cr | 0.79 mg/dL | PIVKA-II | 10 mAU/mL | ||
| CRP | 9.6 mg/dL | T-bil | 5.25 mg/dL | ||
| CA19-9 | 193 784.3 U/mL | Amylase | 11 U/L | ||
| CA125 | 91.6 U/mL | ||||
| CEA | 2.1 ng/mL | Cytology | class II | ||
| AFP | 3.0 ng/mL | ||||
| PIVKA-II | 19 mAU/mL |
| Complete blood count . | . | Biochemistry . | . | Abdominal fluid . | . |
|---|---|---|---|---|---|
| WBC | 4600/μL | T-bil | 0.81 mg/dL | Color | Dark red |
| Hb | 10.2 g/dL | AST | 18 U/L | CA19-9 | 2 672 400 U/mL |
| Plt | 33.9 × 104/μL | ALT | 20 U/L | CA125 | 2618.9 U/mL |
| ALP | 74 U/L | CEA | 41.7 ng/mL | ||
| γ-GTP | 18 U/L | AFP | 1.4 ng/mL | ||
| Cr | 0.79 mg/dL | PIVKA-II | 10 mAU/mL | ||
| CRP | 9.6 mg/dL | T-bil | 5.25 mg/dL | ||
| CA19-9 | 193 784.3 U/mL | Amylase | 11 U/L | ||
| CA125 | 91.6 U/mL | ||||
| CEA | 2.1 ng/mL | Cytology | class II | ||
| AFP | 3.0 ng/mL | ||||
| PIVKA-II | 19 mAU/mL |
From these findings, we made the diagnosis of ruptured simple liver cyst and performed laparoscopic liver cyst fenestration and irrigation drainage 1 week after his admission to our hospital. A large hepatic cyst was found from the right abdomen to the right diaphragm. A dark red fluid suspected to be the content of the ruptured cyst was observed. We perforated the cyst capsule and 2500 ml of bloody fluid was drained. We resected the cystic wall as much as possible (Fig. 4). Postoperatively, paralytic ileus occurred but it was treated conservatively. The patient was transferred back to the previous hospital for the further treatment of the joint fracture on postoperative day 24.
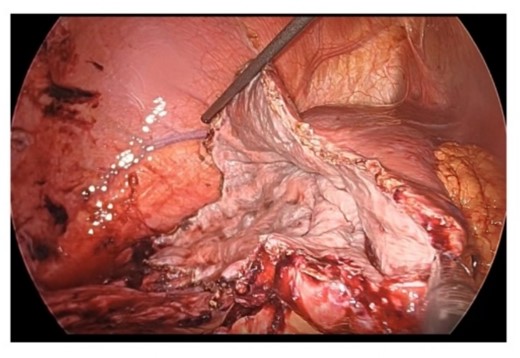
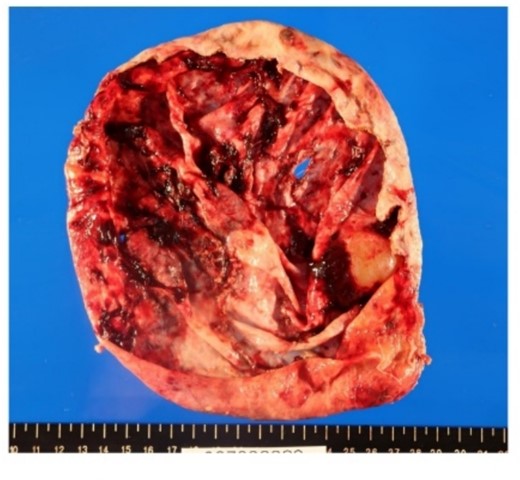
Resection in the cystic wall, remaining 2 cm on the liver side was left.
Surgical specimen showed no neoplastic lesion (Fig. 5). Pathological diagnosis was a non-parasitic simple hepatic cyst with acute inflammation and hemorrhage. The cystic wall presented bile duct epithelium and liver lobules. Immunohistochemical staining showed that only CA19-9 was positive in the hepatic cyst wall epithelium (Fig. 6). The patient’s serum levels of CA19-9, CA125 and CEA were 164.0 U/mL, 126.3 U/mL, and 1.0 ng/mL, respectively, on postoperative day 23. A follow-up abdominal CT scan performed 2 months after the surgery did not show any sign of tumor.
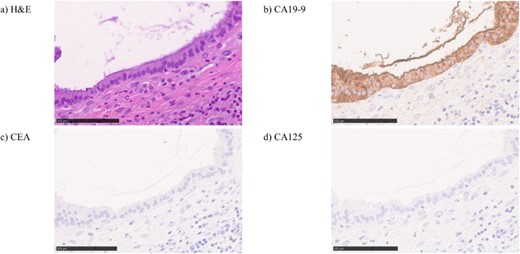
In hematoxylin eosin staining (a), pathological findings of the cystic wall presented bile duct epithelium and liver lobules. Immunohistochemical, the hepatic cyst wall epithelium was only CA19-9 (b) positive, negative for CEA (c) nor CA125 (d).
Discussion
Most simple hepatic cysts are asymptomatic and observation is the standard strategy. Spontaneous rupture of hepatic cysts is sometimes seen in Echinococcus infections, but nonparasitic spontaneous rupture is extremely rare [1]. In case of ruptured simple hepatic cysts, the treatment such as fenestration is required. In our case, we found elevated tumor markers in both of the serum and ascites. We do acknowledge that fenestration or aspiration is contraindicated for the malignant cystic tumors. However, imaging studies with CT and MRI did not show any findings suggesting malignancy such as wall thickening, calcification, cystic fullness, multilocular pattens, substantial component within the cyst or contrast effect. Cytology of the ascites was also negative for cancer. In addition, the cyst had already been ruptured, we performed laparoscopic fenestration.
The CA19-9 level in cystic fluid was reported as a helpful marker for the diagnosis of cystadenoma and cystadenocarcinoma [2]. An elevated CA19-9 level cannot be a diagnostic help for malignancy when the cyst has ruptured [3]. In fact, the serum CA19-9 level rapidly decreased postoperatively in our case. The elevated CA19-9 value in the content fluid of the liver cyst is often encountered and it is known that simple hepatic cysts can produce biological markers such as CA 19–9 and CEA [4, 5]. In our case, immunohistochemical staining showed CA19-9 was positive in the hepatic cyst wall epithelium. We suspected that spillage of a certain number of epithelial cells that were positive for CA19-9 into the abdominal cavity by rupture might cause the elevation of serum CA19-9 level.
To the best of our knowledge, there were seven cases of simple hepatic cysts with elevated serum CA19-9 or cystic fluid CA19-9 levels were reported in the literature including the present case (Table 2). Among the five cases treated with hepatic cyst fenestration, serum CA19-9 levels had been measured after treatment in four cases, and in all the cases it decreased. In all cases, CA19-9 was higher in cystic fluid than in serum. This could support our above mentioned hypothesis that the cyst was the main cause of elevated serum CA19-9. In cases of postoperative CA19-9 elevation, probably because a different cyst from the one that spontaneously ruptured preoperatively ruptured due to the surgical manipulation and leaked intracystic fluid [3]. This report also suggests that intracystic fluid leaking into the abdominal cavity is the primary cause of elevated serum CA19-9. The present case suggests that elevated serum tumor markers does not necessarily imply malignant liver cystic tumor especially in the cases with rupture.
Cases of simple hepatic cysts with elevated serum or cystic fluid CA19-9 levels
| . | Age . | Sex . | Treatment . | Serum CA19-9 (U/mL) . | Cystic fluid CA19-9 (U/mL) . | Serum CA19-9 (U/mL) . |
|---|---|---|---|---|---|---|
| (after treatment) . | ||||||
| Yamaguchi M, et al. [3] | 61 | Male | Fenestration | 151 | >50 000 | 229 (Day 30) |
| Horsmans Y, et al. [6] | 69 | Female | 28 | 370 000 | ||
| Sato T, et al. [7] | 57 | Male | Fenestration | 661.3 | 12 000 000 | 70 (Day 14) |
| Yanai H, et al. [8] | 76 | Female | Minocycline hydrochloride injection | 170 | 371 | 110 (Day 7) |
| Takahashi G, et al. [9] | 59 | Male | Fenestration | 48.3 | ||
| Zurli L, et al. [10] | 67 | Female | Fenestration | 3287 | 2 763 420 | |
| Our case | 72 | Male | Fenestration | 193 784.3 | 2 672 400 | 164 (Day 23) |
| . | Age . | Sex . | Treatment . | Serum CA19-9 (U/mL) . | Cystic fluid CA19-9 (U/mL) . | Serum CA19-9 (U/mL) . |
|---|---|---|---|---|---|---|
| (after treatment) . | ||||||
| Yamaguchi M, et al. [3] | 61 | Male | Fenestration | 151 | >50 000 | 229 (Day 30) |
| Horsmans Y, et al. [6] | 69 | Female | 28 | 370 000 | ||
| Sato T, et al. [7] | 57 | Male | Fenestration | 661.3 | 12 000 000 | 70 (Day 14) |
| Yanai H, et al. [8] | 76 | Female | Minocycline hydrochloride injection | 170 | 371 | 110 (Day 7) |
| Takahashi G, et al. [9] | 59 | Male | Fenestration | 48.3 | ||
| Zurli L, et al. [10] | 67 | Female | Fenestration | 3287 | 2 763 420 | |
| Our case | 72 | Male | Fenestration | 193 784.3 | 2 672 400 | 164 (Day 23) |
Cases of simple hepatic cysts with elevated serum or cystic fluid CA19-9 levels
| . | Age . | Sex . | Treatment . | Serum CA19-9 (U/mL) . | Cystic fluid CA19-9 (U/mL) . | Serum CA19-9 (U/mL) . |
|---|---|---|---|---|---|---|
| (after treatment) . | ||||||
| Yamaguchi M, et al. [3] | 61 | Male | Fenestration | 151 | >50 000 | 229 (Day 30) |
| Horsmans Y, et al. [6] | 69 | Female | 28 | 370 000 | ||
| Sato T, et al. [7] | 57 | Male | Fenestration | 661.3 | 12 000 000 | 70 (Day 14) |
| Yanai H, et al. [8] | 76 | Female | Minocycline hydrochloride injection | 170 | 371 | 110 (Day 7) |
| Takahashi G, et al. [9] | 59 | Male | Fenestration | 48.3 | ||
| Zurli L, et al. [10] | 67 | Female | Fenestration | 3287 | 2 763 420 | |
| Our case | 72 | Male | Fenestration | 193 784.3 | 2 672 400 | 164 (Day 23) |
| . | Age . | Sex . | Treatment . | Serum CA19-9 (U/mL) . | Cystic fluid CA19-9 (U/mL) . | Serum CA19-9 (U/mL) . |
|---|---|---|---|---|---|---|
| (after treatment) . | ||||||
| Yamaguchi M, et al. [3] | 61 | Male | Fenestration | 151 | >50 000 | 229 (Day 30) |
| Horsmans Y, et al. [6] | 69 | Female | 28 | 370 000 | ||
| Sato T, et al. [7] | 57 | Male | Fenestration | 661.3 | 12 000 000 | 70 (Day 14) |
| Yanai H, et al. [8] | 76 | Female | Minocycline hydrochloride injection | 170 | 371 | 110 (Day 7) |
| Takahashi G, et al. [9] | 59 | Male | Fenestration | 48.3 | ||
| Zurli L, et al. [10] | 67 | Female | Fenestration | 3287 | 2 763 420 | |
| Our case | 72 | Male | Fenestration | 193 784.3 | 2 672 400 | 164 (Day 23) |
Acknowledgements
All authors were thankful for the doctors of Miyamoto Rena (Department of Orthopedic Surgery, Saitama Sekishinkai Hospital, Japan).
Conflict of interest statement
None declared.
Funding
None declared.



