-
PDF
- Split View
-
Views
-
Cite
Cite
Leonora E Long, Sam Tharwat Alhayo, Michael Talbot, Iatrogenic eventration of the hemidiaphragm in a post-bariatric surgery patient, Journal of Surgical Case Reports, Volume 2024, Issue 3, March 2024, rjae117, https://doi.org/10.1093/jscr/rjae117
Close - Share Icon Share
Abstract
The aim of this report is to describe the management of an iatrogenic diaphragmatic eventration following surgery to relieve neurogenic symptoms of thoracic outlet syndrome in a patient with a prior history of sleeve gastrectomy. We discuss the case of a 46-year-old woman with a 6-month history of gastro-oesophageal reflux and dyspnoea. Imaging demonstrated a left hemidiaphragm eventration and hiatus hernia. The patient underwent laparoscopic plication of the left hemidiaphragm, repair of the hiatus hernia, and an omega loop gastric bypass, with satisfactory resolution of her symptoms. This demonstrates that surgical diaphragmatic plication has good outcomes in cases where the abdominal anatomy is already altered as a result of previous bariatric surgery, and that concurrent hiatus hernia repair, plication of iatrogenic diaphragm eventration, and sleeve to gastric bypass conversion satisfactorily relieve reflux and dyspnoea in a morbidly obese patient.
Introduction
The role of the diaphragm is multifaceted. When contracted, it reduces intrathoracic pressure and facilitates lung expansion. In healthy patients without chronic airway disease, it also expands the lower chest wall, further enhancing inspiratory function [1]. Patients with unilateral diaphragmatic paralysis may be unaware of their condition because the contralateral diaphragm and lung may compensate and contribute to overall adequate gas exchange. Diaphragmatic paralysis may also be severe and necessitate mechanical ventilation. Symptoms of diaphragmatic eventration may include intraabdominal pain and digestive problems due to structural changes in the abdominal cavity and increased intra-abdominal pressure.
The diaphragm is innervated by the phrenic nerve, which originates from cervical spinal roots C3, C4, and C5 and runs anterior to the anterior scalene muscle, travelling anteriorly to the subclavian artery before entering the thorax via the superior thoracic aperture.
Diaphragmatic plication is a surgical approach to iatrogenic diaphragmatic paralysis with low morbidity and mortality in adults [2] that produces symptom relief and objective improvement in respiratory function [3] and can allow patients to be weaned from mechanical ventilation [2, 4].
Case presentation
A 41-year-old woman presented with reflux, vomiting, and breathlessness. She had previously undergone laparoscopic sleeve gastrectomy (LSG), resulting in 50 kg of weight loss from an original body mass index (BMI) of 56.8, but had returned to a body weight of 170 kg by the time of consultation. The surgical history also included appendicectomy and cholecystectomy. She had no other comorbidities, was an ex-smoker, and had a high social alcohol intake. She had stopped her work in elderly and disability care due to troublesome dyspnoea.
One year prior to our consultation, the patient had sustained a workplace injury leading to C5/C6 disc damage and left thoracic outlet syndrome. She had trialled medical therapy to resolve neurogenic symptoms before proceeding to surgical intervention involving internal fixation of her lower cervical spine, left first rib resection, and scalenectomy with brachial plexus neurolysis. In Day 1 postoperative chest X-ray (CXR) showed an elevated left hemidiaphragm (Fig. 1a) that was persistent on computed tomography (CT) imaging 1 month later (Fig. 1b; see Video 1), along with complete left basal collapse and lingular atelectasis. This suggested paralysis of the left hemidiaphragm, likely due to a left phrenic nerve injury, with a resulting loss of 60% of lung volume and displacement of the mediastinal structures to the right. The patient had a trial of conservative management. However, increasing reflux and dyspnoea symptoms warranted gastroscopy and volumetric CT that showed an acute angularis angulation of the sleeve (Fig. 1c) and a large gastric volume of 495 ml, in addition to an ongoing high-riding diaphragm on the left side.
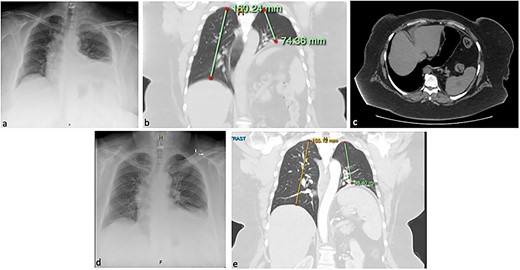
Chest X-ray (CXR; a) and computed tomography (CT) of the chest and abdomen in coronal (b) and axial (c) planes, showing eventration of the left hemidiaphragm and angulation of the stomach. Resolution of eventration on CXR at one day post-plication on CXR (d) and on CT at six months post-plication (e).
We therefore performed laparoscopic Roux-en-Y gastric bypass (RYGB), hiatal repair, and diaphragmatic plication. The technique involved crural dissection, division of the phreno-oesophageal ligament, and then opening of the left pleura for left-sided thoracic insufflation. The primed left diaphragm was then plicated medial to lateral using a non-absorbable barbed suture (Fig. 2; see Video 2). Hiatal repair was performed with posterior cruroplasty and oesophagopexy. A transected lesser curve gastric pouch was created by dividing the previous sleeve at the angularis. A 100/60 cm antecolic Roux/biliopancreatic limb configuration was constructed via hand-sewn gastroenterostomy and stapled enteroenterostomy. Finally, the internal hernia defects were closed. No drains were inserted.
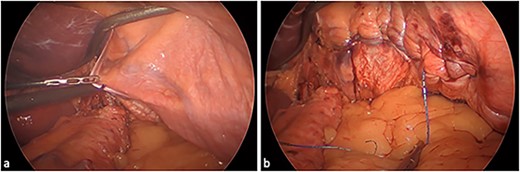
Eventrated left hemidiaphragm: (a) unrepaired and (b) post medial-to-lateral plication.
In Day 1 postoperative CXR showed a normal-riding left diaphragm (Fig. 1d). The patient was discharged home on postoperative Day 3, following an unremarkable and satisfactory recovery. At 6-week follow-up, she was tolerating a normal diet, had no respiratory symptoms or reflux, and had reduced her weight to 128 kg. Six months postoperatively, she remained free of respiratory symptoms and was tolerating a diet. Her weight remained stable. She underwent a gastroscopy that showed that the hiatus hernia repair was intact and there was no evidence of stenosis. A barium swallow showed normal oesophageal anatomy and transit time, and a CT scan, performed to investigate mild dysphagia, showed enlarged left lung volume and normal anatomical configuration of the stomach (Fig. 1e; see Video 3).
Discussion
The phrenic nerve is susceptible to damage during first rib resection because of its anatomic proximity. The left hemidiaphragm was elevated on CXR several hours after the resection; however, this had been attributed to basal atelectasis. Diaphragmatic eventration was not suspected until 8 months later, when the patient underwent gastroscopy and gastric/abdominal 3DCT to investigate symptoms of post-LSG reflux and dyspnoea. While diaphragmatic strength may improve spontaneously [5], our patient’s symptoms became increasingly troublesome preoperatively, warranting surgical treatment. Her reflux was likely due to poor sleeve configuration, in turn attributable to poor diaphragmatic function, resulting in a high-riding left diaphragm and consequently migratory gastric conduit, leading to angulation and poor clearance.
Multiple transthoracic and transabdominal approaches to plication are described, which incorporate open, laparoscopic, thoracoscopic, or robotic techniques and all result in improved symptoms and lung function tests [3]. Laparoscopic plication enables single-lumen endotracheal ventilation, concurrent abdominal intervention, and a more capacious operating field, and minimizes the risk of postoperative intercostal nerve pain that could occur from a thoracic approach [6]. The transthoracic approach may be favourable in patients with a higher BMI or who have undergone previous intra-abdominal surgery [3]; however, the laparoscopic approach in our case revealed no added difficulty compared with usual bariatric surgery. The relief in the patient’s dyspnoea was likely due to the increased lung volume and abolition of paradoxical upward diaphragm movement resulting from the plication. Furthermore, the patient’s reflux symptoms and weight gain were addressed concurrently with a hiatal hernia repair and RYGB. Concurrent diaphragmatic patch and plication may reduce the recurrence of eventration [7], with this combination used more frequently for iatrogenic or pathological eventration than in patients with congenital or traumatic aetiology. However, while reduced diaphragm compliance in the setting of diaphragmatic hernia repair is not a reported concern [8], permanent mesh infection or erosion is a theoretical risk in the context of concurrent RYGB, hence patch repair was not utilized in our case.
Conclusion
To our knowledge, this is the first case to describe concurrent bariatric surgery and laparoscopic diaphragmatic plication, with satisfactory relief of symptoms of reflux and dyspnoea.
Conflict of interest statement
None declared.
Funding
None declared.
Author contributions
Prof Talbot and Dr Alhayo performed the surgery and assisted with writing and revising the manuscript, while Dr Long performed review of the case notes and investigation results as well as wrote the original manuscript.
Consent for publication
The subject provided written informed consent for the publication of the study data.



