-
PDF
- Split View
-
Views
-
Cite
Cite
Alvin Yuan Liang Ng, Michael Gale, Bryce Renwick, Paul Bachoo, Gallbladder necrosis and small bowel ischaemia following fenestrated endovascular aneurysm repair for juxtarenal abdominal aortic aneurysm: a case report, Journal of Surgical Case Reports, Volume 2020, Issue 3, March 2020, rjaa046, https://doi.org/10.1093/jscr/rjaa046
Close - Share Icon Share
Abstract
Anatomical variation may result in unexpected complications after fenestrated endovascular aneurysm repair (FEVAR). We report a 78-year-old gentleman who was admitted for elective FEVAR procedure for a juxtarenal abdominal aortic aneurysm. Three days post-operatively, he deteriorated clinically. Computed tomography (CT) angiogram showed small bowel ischaemia and a replaced right hepatic artery originating from superior mesenteric artery. A necrotic gallbladder found during laparotomy required cholecystectomy following small bowel resection that required a relook for anastomosis and drainage of bile collection. He had prolonged ICU stay requiring treatment for multiple organ dysfunction then spent 4 weeks in hospital. Following multidisciplinary team approach in management of his complications during post-operative phase, he recovered well enough for rehabilitation and discharge home. Surveillance CT aorta at 1 month and 6 months post FEVAR showed satisfactory FEVAR appearance with no endoleak.
INTRODUCTION
Open repair has been the gold standard for the treatment of juxtarenal abdominal aortic aneurysms (AAAs) due to limited commercially available devices for standard endovascular aneurysm repair (EVAR) in treating juxtarenal AAAs [1]. Following improvement in technology, operative technique and being more widely available, fenestrated EVAR (FEVAR) plays an increasingly important role in their treatment.
However, FEVAR has additional complications involving visceral vessels incorporated in the repair compared to EVAR. Although intestinal ischemia has been a recognized complication, gallbladder necrosis has only been reported by Pol et al. [2] post EVAR. The authors highlighted that unexpected complications may occur in the presence of anatomical arterial variations. We reported the first case of gallbladder necrosis after FEVAR with right replaced hepatic artery (RRHA) originating from superior mesenteric artery (SMA), a similar anatomical variant highlighted by Pol et al.
CASE REPORT
A 78-year-old gentleman admitted for elective FEVAR to treat his CT confirmed 5.7 cm juxtarenal AAA. He was not suitable for open repair and initially offered the option of no intervention due to ongoing comorbidities resulting in poor cardiopulmonary function; however, he was keen to proceed with surgery. From pre-implant planning CT angiogram (CTA), infrarenal endograft was unsuitable for EVAR due to reverse conicity and unhealthy infrarenal aortic neck (Fig. 1); therefore, FEVAR was chosen. RRHA from SMA was also seen in pre-operative CTA but not reported (Fig. 2).
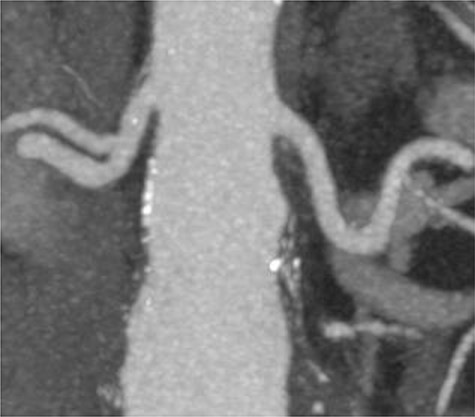
Pre-implant planning CTA showing unhealthy infrarenal aortic neck.
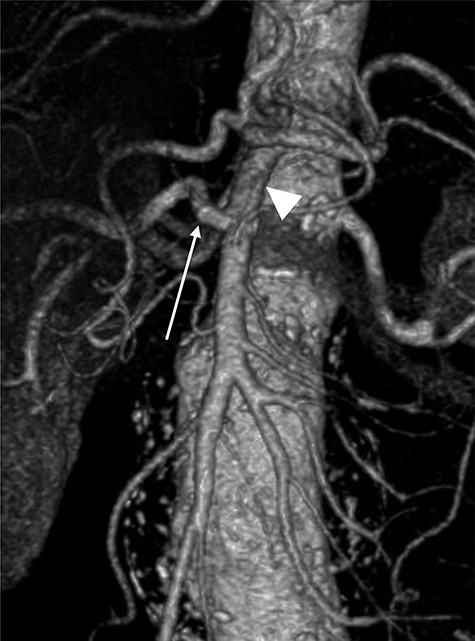
Pre-operative CTA showing healthy SMA (arrowhead) with RRHA (arrow).
Terumo Aortic Anaconda three-vessel fenestrated and bilateral iliac branched graft was used for repair and was deployed in perirenal position with stents to the SMA and both renal arteries. Surgical arterial access was via left axillary artery (LAA) and percutaneous access was ultrasound-guided via common femoral arteries (CFA) bilaterally. Cannulation of left renal artery (V12 5 × 22 mm) and SMA (V12 9 × 32 mm) was via CFA and right renal artery (V12 5 × 22 mm) was through LAA. There was intra-operative difficulty in SMA cannulation, which was eventually cannulated after repositioning of graft (Fig. 3). Final intra-operative angiogram showed patent renal arteries bilaterally, SMA and common iliac arteries bilaterally (Fig. 4). Post-operatively, he was transferred to surgical high dependency unit (HDU). Three days post FEVAR, he developed abdominal pain, haematemesis and melaena with significantly raised inflammatory markers. CT mesenteric angiogram showed an ischaemic small bowel segment. A necrotic gallbladder was found incidentally during laparotomy, which required cholecystectomy after 20 cm segment of small bowel had been resected and decision was made to not anastomose the small bowel due to the intra-operative findings. A relook laparotomy was performed for small bowel anastomosis and drainage of bile collection found in the gallbladder fossa. Histology results later confirmed infarcted gallbladder with extensive acute inflammation as well as congested, ischaemic and focally infarcted small bowel segment with peritonitis.
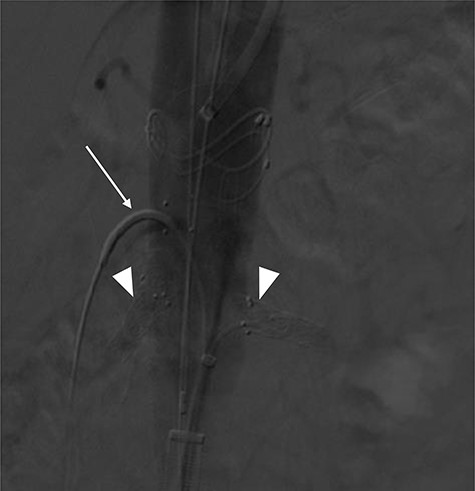
Mid-implantation angiogram showing both renal stents deployed (arrowheads) and SMA cannulated (arrow).
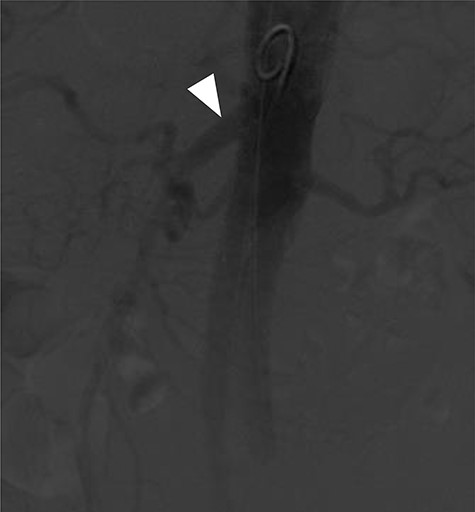
Completion angiogram showing good filling of proximal SMA (arrowhead), renal arteries and common iliac arteries.
Although this was a planned elective FEVAR, several complications requiring two laparotomies, multi-organ dysfunction as well as patient’s multiple comorbidities contributed to prolonged intensive care unit (ICU) stay and delayed discharge. However, a multidisciplinary approach involving relevant specialties had allowed him to make a good recovery for rehabilitation and discharge home. Surveillance CT aorta at 1 month and 6 months post FEVAR showed satisfactory FEVAR appearance with no endoleak (Fig. 5).
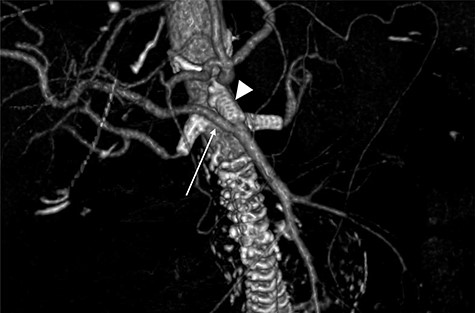
Follow-up CTA showing all visceral stents patent and RRHA (arrow) from SMA (arrowhead).
DISCUSSION
AAAs pose a substantial clinical burden, and a significant proportion of AAAs are unsuitable for open repair or EVAR. EVAR was unsuitable in this case due to AAA neck morphology with poor proximal sealing zone. As fenestrated stent grafts play an increasingly important role in the treatment of juxtarenal AAAs, awareness of complications helps clinicians to take necessary precaution to help reduce any unanticipated consequences.
Although other factors in this case such as difficulty in SMA cannulation and pre-existing significant mural thrombus with extensive aortic and iliac artery calcification could have potentially contributed to significant micro-embolic ischaemic injury to organs supplied by SMA, this anatomical variant has been shown to increase risk for gallbladder necrosis. Pol et al. [2] described a case of gallbladder necrosis secondary to EVAR, which was likely due to RRHA arising from SMA. Similarly in this case, gallbladder necrosis was identified secondary to FEVAR with the same anatomical variant. In the literature, RRHA arising from SMA is the most common hepatic artery variation. This anatomical variant has been reported up to as high as 11–21% by Shukla et al. [3] in a systematic review.
When reviewing the significance of anatomical variants, Biehl et al. [4] described the importance of identifying RRHA in laparoscopic cholecystectomy to prevent vascular or biliary damage especially if it runs anterior to common hepatic duct. Literatures have also shown that recognition of such aberrations in vascular anatomy is essential to surgeons performing pancreaticoduodenectomy (PD) as well as in prospective transplant donors and recipients for the proper management of living donor liver transplantation, as transplantation of the right lobe is heavily favoured over the left. This aberration affects the safety of both donor and recipient [5].
However, not many literatures have discussed about the significance of identifying these anatomical variants in endovascular procedures. Pre-operative imaging studies will often be adequate to identify these anomalies, but interpreting radiologists may not be aware of its clinical significance. Stauffer et al. [6] reported that radiologists in their institution now routinely describe the hepatic arterial anatomy of patients with pancreatic malignancy for PD planning to avoid vessel damage during dissection. Following the case reported by Pol et al. and this case resulting in gallbladder necrosis due to a similar anatomical variant, it does pose a question whether routine reporting of this anatomical variant during pre-operative planning in endovascular procedure is required.
Another aspect worth highlighting in this case is the importance of a multidisciplinary team approach to improve outcome for critically ill high-risk surgical patients by bringing together a group of experts who can independently contribute towards diagnosis and provide individually tailored treatment strategy [7].
Conclusion
Gallbladder necrosis is a potential complication that occurs in both EVAR and FEVAR with RRHA arising from SMA as demonstrated in these cases. Although this complication may not be completely preventable, consideration for routine reporting of this anatomical variant in pre-operative planning may be considered. The awareness of this complication during endovascular procedure due to this anatomical variant can reduce the risk of unanticipated consequences.
Conflict of interest statement
There are no reported conflicts of interest for this paper.
References
- abdominal aortic aneurysm
- angiogram
- aorta
- computed tomography
- cholecystectomy
- repair of aneurysm
- anastomosis, surgical
- bile fluid
- intensive care unit
- laparotomy
- superior mesenteric artery
- necrosis
- patient care team
- gallbladder
- rehabilitation
- multiple organ dysfunction syndrome
- small-intestine resection
- surveillance, medical
- small bowel ischemia
- right branch of hepatic artery
- interdisciplinary treatment approach
- endoleak
- fenestration
- gallbladder necrosis



