-
PDF
- Split View
-
Views
-
Cite
Cite
Amr Meselhi, Elsamoual Mohammed, Simon Ray-Chaudhuri, A typical etiology of a common carotid artery aneurysm: diagnostic and therapeutic insights from a case report, Journal of Surgical Case Reports, Volume 2025, Issue 8, August 2025, rjaf658, https://doi.org/10.1093/jscr/rjaf658
Close - Share Icon Share
Abstract
Carotid artery aneurysms are rare, accounting for only 0.4% to 4% of all peripheral artery aneurysms, and can present with non-specific symptoms. We report a case of a 38-year-old male who presented with a 2-week history of a tender, pulsatile left neck mass. He had no history of trauma, infection, or connective tissue disease, but was an active smoker. Clinical examination and contrast-enhanced CT angiography revealed a 2.1 cm aneurysm of the left common carotid artery. The patient underwent successful open surgical repair using a reversed great saphenous vein interposition graft. Histopathological examination of the aneurysm wall and a comprehensive vasculitis workup were unremarkable. The patient had an uneventful postoperative course and remained asymptomatic on follow-up. This case highlights the importance of early imaging and surgical intervention in managing carotid artery aneurysms to prevent potentially catastrophic complications.
Introduction
Extracranial carotid artery aneurysms (ECAAs) are rare vascular anomalies, comprising <1% of all arterial aneurysms and ~0.4%–4% of peripheral artery aneurysms [1, 2]. These aneurysms can arise from various etiologies, including atherosclerosis, trauma, infections, and connective tissue disorders [3, 4]. True aneurysms are typically linked to degenerative or inflammatory processes, whereas pseudoaneurysms more commonly result from trauma or iatrogenic injury [5].
Clinically, patients may be asymptomatic or present with a pulsatile cervical mass, local compressive symptoms, or thromboembolic events such as transient ischemic attacks or stroke [4, 6]. The rarity of ECAAs contributes to the absence of standardized treatment protocols; however, there is a general consensus favoring intervention for symptomatic aneurysms or those >2 cm in diameter [3, 6].
Imaging, particularly CT angiography, plays a pivotal role in diagnosis and surgical planning, providing detailed visualization of aneurysm morphology and arterial anatomy [7]. While both open and endovascular repair techniques are available, open surgery remains the preferred approach for true aneurysms, especially in anatomically complex cases or younger patients [8, 9].
This report presents a rare case of an idiopathic left common carotid artery aneurysm in a young adult without typical risk factors, managed successfully with open surgical repair. We aim to contribute to the limited literature on non-atherosclerotic carotid aneurysms and discuss the diagnostic and therapeutic considerations in such unusual presentations.
Case history
A 38-year-old male presented to the emergency department with a 2-week history of a tender left-sided neck mass. He also reported a headache but had no neurological symptoms.
His past medical history included schizophrenia (managed with medication), recurrent lower limb deep vein thrombosis (DVT), and a significant history of heavy smoking. Initial laboratory investigations were unremarkable except for an elevated C-reactive protein level of 50 mg/L.
On physical examination, vital signs were stable. A tender, pulsatile mass was palpable in the left anterior triangle of the neck (Fig. 1). Ultrasound revealed a 21 × 16 mm aneurysm of the left common carotid artery (CCA) with prominent reactive cervical lymphadenopathy. This was further evaluated with CT angiography, which confirmed a left CCA aneurysm involving the carotid bulb (Fig. 2).
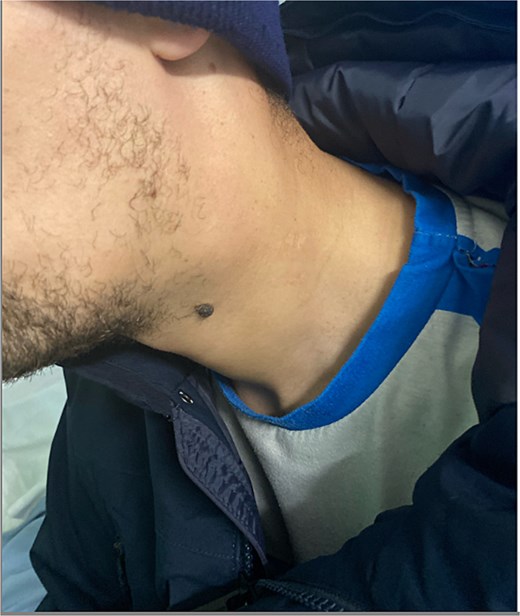
Clinical image showing a left-sided neck swelling in the anterior triangle.
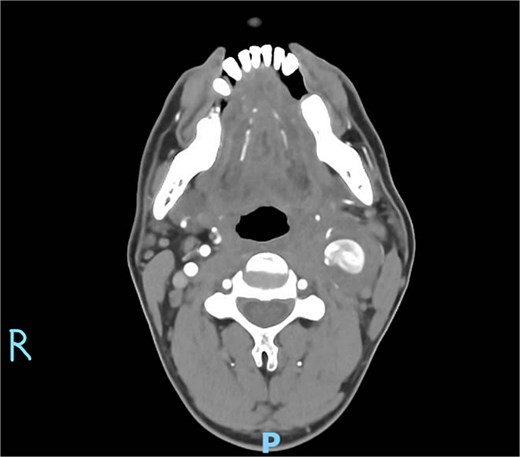
Axial CT angiography demonstrating the left common carotid artery aneurysm.
Intraoperatively, a standard cervical approach was used to expose the carotid bifurcation. The aneurysm was carefully dissected from surrounding structures, as shown in Fig. 3, which demonstrates the involvement of the CCA, internal carotid artery (ICA), and occluded external carotid artery (ECA). After systemic heparinization, proximal and distal control of the CCA and ICA was established. The aneurysm, which had a fusiform and inflamed appearance, was visualized and opened (see Fig. 4). It was then completely resected. A reversed great saphenous vein (GSV), harvested from the leg, was used as an interposition graft between the CCA and ICA. The ECA, which was already occluded and not amenable to reconstruction, was ligated. The completed anastomosis and interposition graft are shown in Fig. 5, confirming restored flow and graft placement. Hemostasis was secured, and a surgical drain was placed before layered closure of the wound.
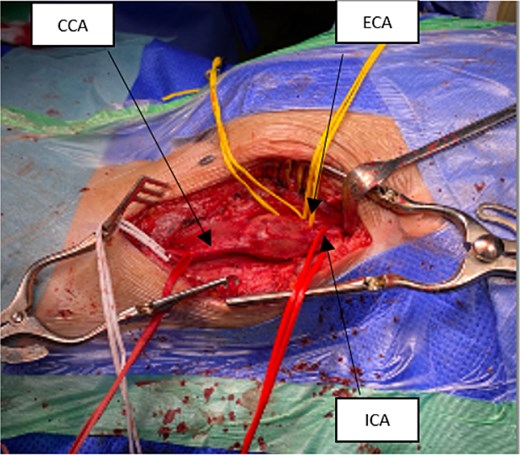
Intraoperative exposure showing aneurysm involving the carotid bulb; visible structures include CCA, ICA, and occluded ECA.
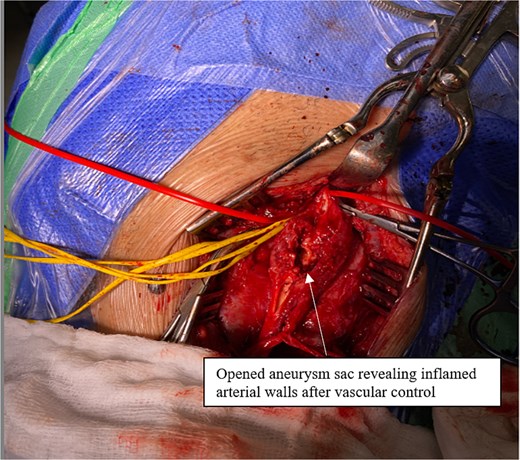
Opened aneurysm sac revealing inflamed arterial walls after vascular control.
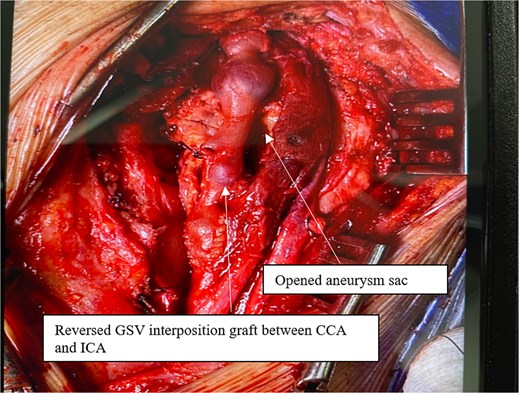
Completed repair with reversed GSV interposition graft between CCA and ICA; ECA is ligated.
In the immediate postoperative period, the patient developed secondary hemorrhage while in recovery. He was intubated and returned urgently to the operating theatre, where bleeding branches were identified and repaired. He was then admitted to the intensive care unit (ICU) for monitoring.
His recovery was otherwise uneventful, and no neurological deficits were observed. A follow-up CT angiogram of the thoracic and abdominal aorta showed no evidence of additional aneurysms or vascular abnormalities. A full vasculitis screen was negative. Histopathological examination of the aneurysm wall revealed necrosis and granulation tissue formation, but no definitive diagnostic features.
The patient was discharged on a 7-day course of oral co-amoxiclav, along with apixaban and aspirin. At his 2-month follow-up, his wound had healed completely, and he had returned to normal daily activities.
Discussion
Carotid artery aneurysms are a rare vascular pathology, accounting for ~0.1%–2% of all carotid procedures and only 0.4%–4% of peripheral artery aneurysms [2, 10]. Etiologies include atherosclerosis, trauma, congenital anomalies, fibromuscular dysplasia, and infections [3]. Although many ECAAs remain asymptomatic, they may present with a pulsatile neck mass, local discomfort, cranial nerve palsies, rupture, or symptoms related to thromboembolism such as transient ischemic attacks or stroke [4].
In a review of 359 patients, Martinez et al. found atherosclerosis to be the most common cause of true ECAAs, with 83% undergoing surgical repair and smaller proportions managed conservatively or with endovascular techniques [5]. Pseudoaneurysms, more often post-traumatic, were managed endovascularly in 55% of cases, suggesting a shift in practice based on aneurysm type.
A systematic review of 959 cases showed that open surgical repair remained the most common treatment (750 patients), though endovascular techniques had fewer perioperative complications [6]. Complication rates with surgery included cranial nerve injury (9%), stroke (4%), and mortality (2%), while endovascular treatment showed promising short-term safety but lacked long-term data.
Attigah et al. proposed a classification system for ECAA based on anatomical location, with our patient falling under Type V: isolated CCA aneurysms [7].
Diagnosis typically involves clinical assessment and imaging. A pulsatile neck mass is the most common sign, present in 93% of cases, while neurologic symptoms occur in ~50% [7]. CT angiography remains the gold standard for surgical planning [8].
Similar to our case, Jaha et al. [11] reported a 70-year-old female patient with a large extracranial ICA aneurysm, presenting with a pulsatile neck mass and transient ischemic symptoms. The aneurysm measured 42 × 31 mm and was managed successfully with open surgical excision and primary end-to-end ICA anastomosis, without the need for graft placement. The patient experienced no postoperative neurological complications and had an excellent recovery at follow-up [11].
Likewise, de Boer et al. [12] described a case of a 67-year-old woman with a 25 × 20 mm extracranial ICA aneurysm, incidentally discovered after transient neurological symptoms. She underwent open surgical resection with direct vessel repair. Postoperative imaging confirmed patency, and the patient remained neurologically intact at 4 months [12].
While our case differs in that the patient was younger and the aneurysm involved the CCA, requiring a reversed GSV interposition graft due to ECA occlusion, these reports reinforce the effectiveness of open surgical repair. They highlight the importance of individualized surgical planning based on vessel anatomy, aneurysm morphology, and intraoperative findings.
Surgical resection with interposition grafting remains the cornerstone of treatment [9]. Although endovascular repair offers a less invasive alternative, it is best reserved for select high-risk patients or anatomically suitable lesions [9]. Our case reaffirms the importance of early diagnosis and appropriate intervention in preventing serious outcomes.
Conflict of interest statement
None declared.
Funding
None declared.
References
- vasculitis
- aneurysm
- physical examination
- connective tissue diseases
- common carotid artery
- follow-up
- surgical procedures, operative
- wounds and injuries
- infections
- diagnosis
- diagnostic imaging
- neck mass
- carotid artery aneurysm
- computed tomographic angiography
- great saphenous vein
- causality
- histopathology tests
- interposition graft
- peripheral artery aneurysm



