-
PDF
- Split View
-
Views
-
Cite
Cite
Devon N Thomas, Armand Asarian, Philip Xiao, Adenoid cystic carcinoma of the breast, Journal of Surgical Case Reports, Volume 2019, Issue 1, January 2019, rjy355, https://doi.org/10.1093/jscr/rjy355
Close - Share Icon Share
Abstract
Adenoid cystic carcinoma of the breast (ACC) is a rare tumor, comprising <0.1% of all breast cancers. It has a unique dual-cell pattern and is indistinguishable from ACC arising from salivary tissue. It is a low-grade tumor with favorable prognosis, and rare metastasis, with unique features. It is more commonly seen in older women with a mean age at diagnosis of 63, with Caucasian women being at greatest risk. Most cases present as a painful, palpable mass in the outer quadrants of the breast, and must be diagnosed via core needle biopsy or surgical excisional biopsy. Although few other cancers resemble ACC it is commonly misdiagnosed. Given the rarity of this cancer, treatment guidelines have yet to be well established. Current treatment is focused around surgical resection, however, there are not specific recommendations for the extent of resection due to the lack of cases to draw from.
INTRODUCTION
Adenoid cystic carcinoma of the breast (ACC) is an extremely rare tumor accounting for <0.1% of all breast cancers diagnosed [1–3]. It is known to have a dual cell population of both luminal and basal cells making it indistinguishable from ACC arising in salivary tissue [1–3]. Most commonly the tumors present as well circumscribed, painful, palpable masses [2–4].
Adding to the rarity of ACC is it’s favorable prognosis despite being a triple negative tumor; estrogen-, progesterone- and human epidermal growth factor-receptor 2 negative [1, 2, 5]. Triple negative tumors are often highly aggressive with some of the worst prognoses. However, ACC is an anomaly with indolent behavior and a 10-year survival rate ranging from 85 to 100% [2, 5].
Unlike most other breast cancers, ACC rarely metastasizes to the axillary lymph nodes, and therefore dissection is not recommended in the management of this disease [3, 6].
CASE REPORT
An 88-year-old African American female presented to an outpatient surgical clinic for a 1-month history of a painful lump in her right breast. She denied any similar events previously and denied ever having a mammogram or breast ultrasound. Her past medical history was unremarkable, and there was no family history of breast or ovarian cancer.
On physical examination the breasts appeared symmetrical with no visible abnormalities. There was a tender mass palpated in the right breast, superior lateral peri-areolar region. No axillary lymphadenopathy was present.
A diagnostic mammogram was performed and found a dense breast with an ill-defined, lobular mass with coarse internal calcifications in the right breast. The right breast in the 7–8 o'clock axis in the retro-areolar region laterally, corresponding to the palpable mass, was found to have a 1.6 cm × 1 cm heterogenous, hypoechoic solid mass with internal coarse calcifications. Also in the right breast 7–8 o'clock axis 3 cm from the nipple was a hypoechoic mass with irregular margins measuring 6 mm × 3 mm. The left breast showed in the 2 o'clock axis 2 cm from the nipple, an irregular solid mass measuring 7 mm × 2.4 mm. The axillary regions were of normal morphology. The mammogram was graded BIRADS 4 for suspicious findings.
Microscopic examination from an ultrasound guided core-needle biopsy from the 7–8 o'clock retro-areolar region shows tumor composed of both luminal and basal cells (small bland myoepithelial cells with scant cytoplasm and dark compact angular nuclei surround pseudoglandular spaces with mucin) (Fig. 1). Immunohistochemical stain p63 positive for basal cells (Fig. 2). Combined with morphological features, this immunoprofile supports the diagnosis adenoid cystic carcinoma. The suspicious mass in the right breast 7–8 o'clock and the left breast 2 o'clock both showed stromal fibrosis and adenosis.
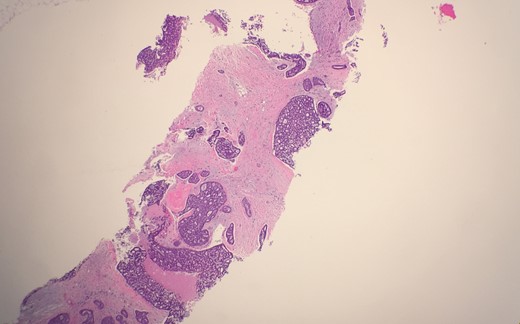
Microscopic examination from an ultrasound guided core-needle biopsy from the 7–8 o'clock retro-areolar region shows tumor composed of both luminal and basal cells (small bland myoepithelial cells with scant cytoplasm and dark compact angular nuclei surround pseudoglandular spaces with mucin).
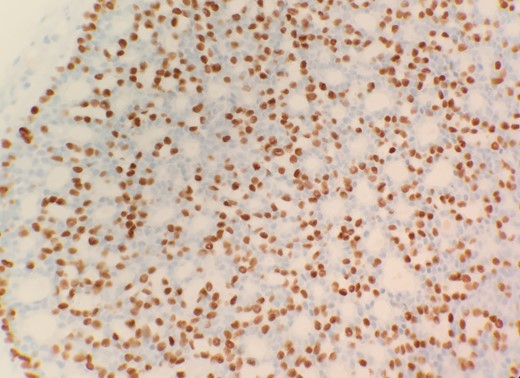
Post core-needle biopsy the patient’s condition was upgraded to BIRADS 6 demonstrating known malignancy.
DISCUSSION
Although there are limited number of cases to draw from, the most common presentation of ACC is the presence of a palpable mass in the upper-outer quadrant of the breast. Some patients report pain on palpation, yet others are asymptomatic and found incidentally on screening mammogram [5].
Once the mass has been found a diagnostic mammogram and/or, a breast ultrasound is warranted. Imaging modalities typically show an irregular mass with spiculated margins often ranging in diameter from 1 to 3 cm. Core needle biopsy is often the next step in diagnosis, however, a tissue diagnosis, post-surgical excision, should be done in order to determine the true nature of the tumor [5].
Diagnostic criteria for ACC requires a biphasic cellular pattern comprised of myoepithelial and epithelial cells [2]. Histologically ACC may resemble invasive ductal carcinoma, which has lead to core biopsy specimens being incorrectly diagnosed [5].
There are few other cancers that could be mistaken for ACC, but collagenous spherulosis (CS) is one of them and must be correctly distinguished for proper diagnosis. The formation of the ductal epithelium is one of the main distinguishing features between it and ACC. In CS it is evident as cellular lobules and forms ductal lumina of various sizes. This is a rare phenomenon to be found in ACC, and if it is present then the ductal lumina formed are much smaller [7].
Cribiform carcinoma, another differential diagnosis, must be ruled out when diagnosing ACC. Both aforementioned cancers exhibit a cribiform pattern histologically [8]. In order to make a correct diagnosis an immunohistochemical stain to examine basement membrane materials or an ultrastructural examination is recommended. A majority of these cancers cannot be accurately identified based solely on the intraoperative frozen section and require further staining and immunohistochemistry to confirm [8].
ACC has been reported in women over a wide age range, but women over the age of 50 years old have an 11× increased risk in developing ACC when compared with younger women.
The mean age at diagnosis is 63 years and Caucasian women are those most affected [2, 5].
Keeping in line with its low-grade and indolent nature, ACC has not been shown to increase the risk for any subsequent solid tumors, or higher-order tumors of the breast [5]. Metastasis from ACC is uncommon, but when it does occur the lungs are most commonly affected with the liver, bones and kidneys following; unsurprisingly these are the same sites of metastasis for ACC of salivary tissue. Extremely rare metastases to the brain have been reported, and axillary lymph node involvement is seen in <2% of patients [9].
Treatment of ACC is currently focused around surgical resection but, there are not clear guidelines for the extent of resection. Some patients have undergone local excision with others receiving radical mastectomies, but there is no evidence to suggest one option over another. However, there have been studies to show an unfavorable increased risk for local recurrence when treated with local excision alone [5].
Due to the low rate of metastasis to the axillary lymph nodes, dissection does not play a beneficial role in the management of ACC.
CONCLUSION
ACC is an extremely rare tumor incompletely understood. It accounts for <0.1% of all breast cancers and although it does not pose a high risk of morbidity or mortality, it still requires more attention in order to formulate better treatment guidelines for clinical management. However, in order for a consensus in management to be established it is necessary to have more cases to learn from.
CONFLICT OF INTEREST STATEMENT
None declared.



