-
PDF
- Split View
-
Views
-
Cite
Cite
Andrew Janssen, Brendan Bych, Johnny Delashaw, Aaron Dumont, Augmented-reality-guided microvascular decompression for trigeminal neuralgia, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf516, https://doi.org/10.1093/jscr/rjaf516
Close - Share Icon Share
Abstract
Augmented reality (AR) allows surgeons to visualize three-dimensional (3D) patient anatomy overlaid onto the surgical field. Procedures such as microvascular decompression, which can be performed without neuronavigation, can benefit from the size, speed, and unique 3D visualizations of this technology. A 78-year-old male presented to neurosurgery clinic with 3 weeks of severe lancinating pain radiating into the right side of his face and jaw. Fast imaging employing steady-state acquisition sequencing showed a dolichoectatic, high-riding, and eccentric right vertebral artery compressing the inferior trigeminal nerve. The decision was made to take the patient for microvascular decompression of the trigeminal nerve. Hoth Intelligence’s AR system was used for preoperative and intraoperative visualization of registered 3D cranial anatomy. This case describes the clinical utility of an AR system for surgical localization and intraoperative visualization during a microvascular decompression surgery. This technology is a feasible alternative to traditional neuronavigation for planning head position, surgical approach, and craniotomy location.
Introduction
Microvascular decompression (MVD) is a neurosurgical procedure designed to alleviate symptoms caused by the compression of cranial nerves, for conditions such as trigeminal neuralgia, hemifacial spasm, and glossopharyngeal neuralgia [1–3]. Traditional methods rely on 2D imaging and the surgeon's anatomical knowledge. It can be both challenging and misleading to interpret detailed interactions between anatomic structures in this context. Augmented reality (AR) represents a major advancement in the field of neurosurgery by overlaying 3D digital anatomic information onto the surgical field of view [4–8]. In this report, we review an anatomically unique case of trigeminal neuralgia that highlights the benefits of using AR in surgical planning and decision-making for MVD procedures. To our knowledge, this is the first reported clinical use of AR for the surgical planning of an MVD procedure.
Case report
A 78-year-old male presented with 3 weeks of severe lancinating pain radiating into the right side of his face and jaw. The pain distribution matched the innervation pattern of the third branch of the trigeminal nerve. These episodes occurred 25–30 times per day, were short-lived, and were rated as severely painful with associated paresthesias. He was started on carbamazepine based on clinical suspicion for trigeminal neuralgia. A noncontrast MRI of the brain with fast imaging employing steady-state acquisition (FIESTA) sequence [9] showed a dolichoectatic, high-riding, and eccentric right vertebral artery compressing the inferior trigeminal nerve (Fig. 1). The decision was made to take the patient for MVD of the trigeminal nerve.
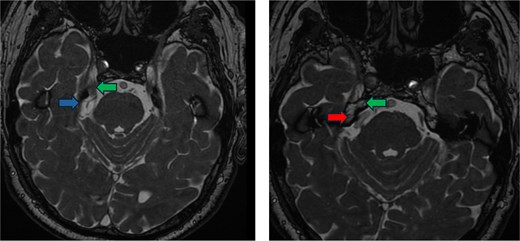
Preoperative imaging. FIESTA scan demonstrating the compression of the trigeminal nerve (green arrow) by the vertebral artery (red arrow) inferiorly and the superior cerebellar artery (blue arrow) superiorly.
3D model generation
A 3D model was generated from the patient’s MRI scan. The following structures were segmented using ITK Snap 3D segmentation software: brain, transverse sinus, sigmoid sinus, basilar artery, vertebral artery, superior cerebellar artery, brainstem, and trigeminal nerves.
Augmented reality technology platform
The AR system (Hoth Intelligence, Philadelphia, PA) operates entirely out of the Microsoft Hololens 2 headset (Redmond, WA). The headset displays digital 3D models superimposed onto the users’ real-world view via an optical see-through display [10, 11]. The system registers the digital model onto the patients’ head using the infrared depth sensors and RBG cameras of the Hololens 2 headset without the need for fiducials.
Operative course
AR visualization of the 3D model demonstrated a laterally and superiorly deviated vertebrobasilar junction with a compressive dolichoectatic and atherosclerotic elbow of the right vertebral artery causing compression as it traversed underneath the trigeminal nerve root entry zone (Fig. 1). A downward-looping superior cerebellar artery was also compressing the nerve from above. In the operating room, the AR system was used to register the 3D model onto the patient’s head, which allowed us to plan the incision and craniotomy location in a more individualized manner and to optimally position the patient for a more ergonomic operation (Fig. 2 and Video 1). The AR overlay displayed the underlying transverse–sigmoid junction, allowing a more accurate burr hole placement. There was a loop of the superior cerebellar artery which was compressing the nerve superiorly at the root entry zone. The vertebral artery was identified. We created a sling with a 4-0 Nurolon suture wrapping around the vertebral artery and suturing it to the dura inferiorly (Video 2). Teflon pledgets were then used to pad the nerve and it was now completely decompressed from the vascular structures. Postoperative MRI of the brain showed effective decompression of the trigeminal nerve (Fig. 3).
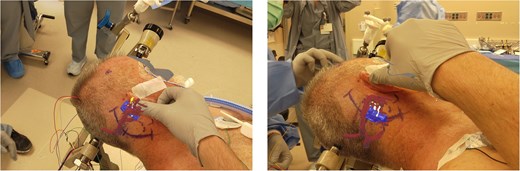
Surgeon’s view through the AR headset of the 3D model registered onto the patient. The model contains vasculature (red), brainstem (blue), and trigeminal nerve (yellow). The surgeon can optimize the head position and plan incision and craniotomy with the view of the registered 3D model.
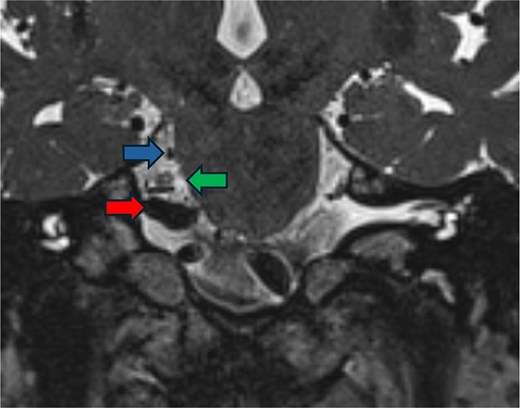
Postoperative imaging. FIESTA scan demonstrating the relief of compression of the trigeminal nerve (green arrow) by the vertebral artery (red arrow) and the superior cerebellar artery (blue arrow).
Discussion
We describe the successful integration of an AR system into the operative workflow of a neuroanatomically complex MVD surgery. To our knowledge, this is the first reported use of AR for operative planning of an MVD surgery. Understanding this patient’s 3D anatomy using AR allowed us to be better prepared for how to decompress the nerve in a relatively uncommon presentation. It provided clarity on the interaction between an aberrant vertebrobasilar system and the compressive vertebral artery at the trigeminal nerve root entry zone. It provided a direct view of the operative retrosigmoid approach, allowing us a more individualized and confident surgical plan. This is in comparison to standard-of-care use of anatomic landmarks, and is significantly faster and has a smaller operating room footprint than traditional neuronavigation.
There are various works exploring the use of AR for surgical planning. These systems are predominantly used outside the operating room, to study the 3D anatomic model [12–14]. However, unlike the mixed reality technologies described in previous reports that only allow visualization of nonregistered 3D models (i.e. floating in space), the AR system used in this case uses a highly efficient process to register patients’ 3D anatomy onto the head. As such, we had more confidence in our approach based on the ability to visualize key landmarks that may be encountered along our operative trajectory, notably the transverse and sigmoid sinuses. The ability to accomplish the same task in a 1.2 lb system with fast registration was a major advantage of the system. This report is significant as it describes a new yet logical use of AR.
Given the patients imaging findings and lack of response to medical management, we felt that MVD was the most reasonable treatment. Intraoperatively, one key step in this procedure involved threading suture under the vertebral artery and the cranial nerve 7/8 complex and then tacking it to the inferolateral dural wall of the skull base to mobilize the artery away from the nerve without kinking it. A literature review found that similar operative techniques have been used by different groups, but many rely on other materials to create the sling. One of these groups cut a dural graft into a cradle shape, draped it around the offending vertebral artery, and then tacked it to the lateral skull base using an aneurysm clip [15]. Another group described a series of cases that used glue-coated Teflon to transpose the vessel in either the anteromedial or posterolateral direction [16].
There are limitations to this report. The quantitative clinical benefit of using AR was not assessed. Impacts on surgical outcome, operative time, and complication rate should be studied in the future. Additionally it would be interesting to compare efficiency and clinical outcomes in MVD cases using traditional neuronavigation versus AR. Lastly, this report describes a single case; therefore, future work evaluating the impact of AR in a larger series of MVD cases is warranted.
The integration of AR in MVD offers promising advancements for enhancing surgical precision by allowing for a more precise localization and 3D understanding of critical structures. This report describes the use of 3D anatomical modeling together with a seamless AR registration process for real-time operative planning. As AR technology continues to evolve, it is likely to become an invaluable tool in neurosurgery.
Conflict of interest statement
None declared.
Funding
None declared.



