-
PDF
- Split View
-
Views
-
Cite
Cite
Emily Harris, Giovanni Giannotti, Is pneumatosis cystoides intestinalis a lymphatic pathology?: A case of small bowel lymphangioma and subsequent development of pneumatosis cystoides intestinalis in a 57-year-old female, Journal of Surgical Case Reports, Volume 2019, Issue 1, January 2019, rjy334, https://doi.org/10.1093/jscr/rjy334
Close - Share Icon Share
Abstract
The etiology of pneumatosis cystoides intestinalis (PCI) is multifactorial and its corresponding treatment is similarly complex. One causation of PCI, is lymphatic disruption. This association is discussed in this case report involving an incident of abdominal lymphangioma and subsequent development of PCI in a 57-year-old female. The purpose of this paper is to further understand the pathology of PCI, specifically as it relates to lymphatic disruption. Furthermore, the purpose of this article is to define a treatment protocol for this disease when it involves lymphatic pathology; i.e. when a surgical vs conservative approach is warranted.
INTRODUCTION
We present a case report of obstructing pneumatosis cystoides intestinalis (PCI) of the proximal jejunum in a 57-year-old woman, with a relevant past surgical history of recent jejunal lymphangioma resection (February 2018). PCI is characterized by the presence of multiple gas-filled cysts in the intestinal wall, the submucosa and/or subserosa of the intestine [1–3]. First described in autopsy by Du Vernoy in 1730 and named by Mayer as cystoides intestinal pneumatosis 1825, its etiology has not been clearly described [2].
PCI is a perturbing finding as it is a clinical sign and is not a clinical diagnosis [4]. The presentation of this disease may be either a benign incidental finding which can be observed; or it may be an ischemic finding with associated perforation, necrosis, sepsis, obstruction [1, 2, 4, 5]. The appropriate therapy depends on the cause of the PCI, with overall mortality to be between 20 and 25% [4].
The presence of gas in the intestinal wall is presumed to be related to two discrete mechanisms: a mechanical theory of gas dissection into the intestinal wall due to mucosal breaks by bacterial forming gas; or from increased intraluminal pressure (i.e. from pulmonary source) causing gas to diffuses across an intact mucosa within the bowel, thus forming cysts [1, 4].
However, lymphatic disruption has been described a third potential cause of PCI [2, 3, 5]. This idea of lymphatics being a primary causative factor of non-toxic appearing episodes of PCI is relevant to our patient as antecedent to her presentation with PCI she underwent a bowel resection of jejunal lymphangioma (February 2018). Abdominal lymphangiomas are a rare benign cystic tumor that can become locally invasive and often require resection [6]. Lymphangioma is a congenital, abnormal embryonic development of lymphatic system causing sequestration of lymphatic tissue [7, 8]. Specifically, this lymphatic congenital abnormality occurs during embryogenesis, in which a default connection between lymphatic vessels and the venous system causes formation of the lymphatic cyst [8]. Therefore, it can be surmised that the jejunal lymphangioma resection in this patient directly contributed to the development of a non-toxic form of PCI in this 57-year-old female.
CASE DESCRIPTION
A 57-year-old non-toxic appearing female with a medical history of diverticulosis and malnutrition, and a surgical history significant for small bowel obstruction due to jejunal lymphangioma status post exploratory laparotomy and resection of bowel (2.2018). This patient presents with epigastric pain ×2 days with radiation to left upper quadrant and nausea with emesis, concerning for obstruction. In the Emergency Department, the patient was non-toxic appearing, hemodynamically stable, with no leukocytosis (7.4), or elevated lactic acid (1.2). Imaging, XR (Fig. 1) and CTAP (Fig. 2), demonstrating SBO with free air concern for perforated viscus. The concern for free air on imaging, in the setting of abdominal pain with symptoms of obstruction, mandated an operative intervention. On 7.5.18 the patient underwent exploratory laparotomy and was found to have emphysema of proximal jejunum, and underwent 125 cm resection of jejunum (Figs 3 and 4). Pathology of the specimen demonstrated multiple air filled cystic serosal nodules consistent with PCI (Figs 3 and 4, Supplementary Fig. S7).
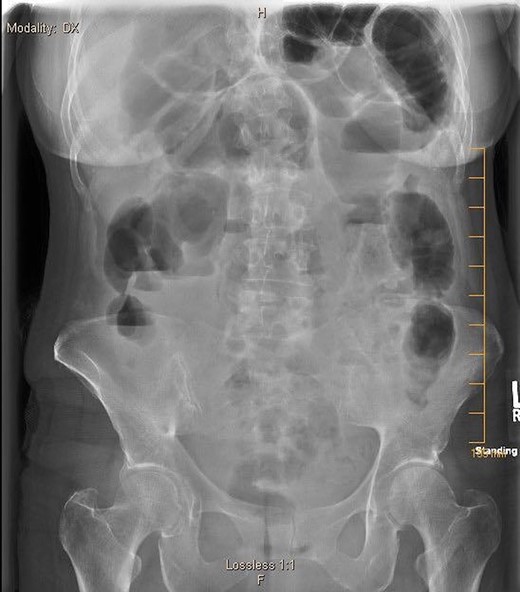
7.4.18: XR abdomen acute complete w/chest: dilated small bowel loops in LUQ, air fluid levels in right mid abdomen, paucity of large bowel gas, suggestive of small bowel obstruction, suggestion of free air.
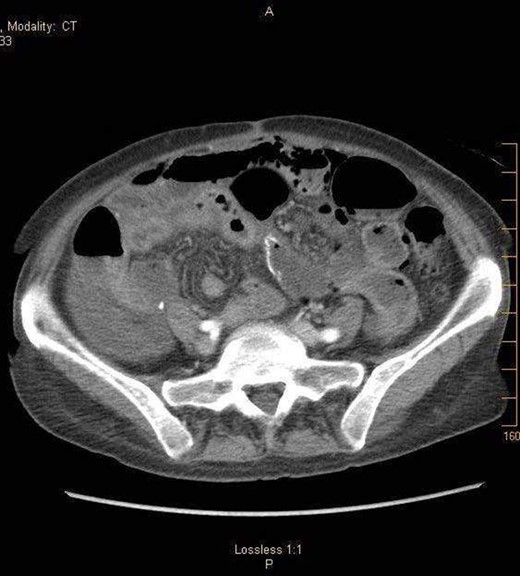
7.4.18:CTAP w/IV and PO contrast: suggestive for small bowel obstruction, distal small bowel collapsed, mild or moderate free air suggestive of perforated viscus, free fluid in dependent portion of pelvis.
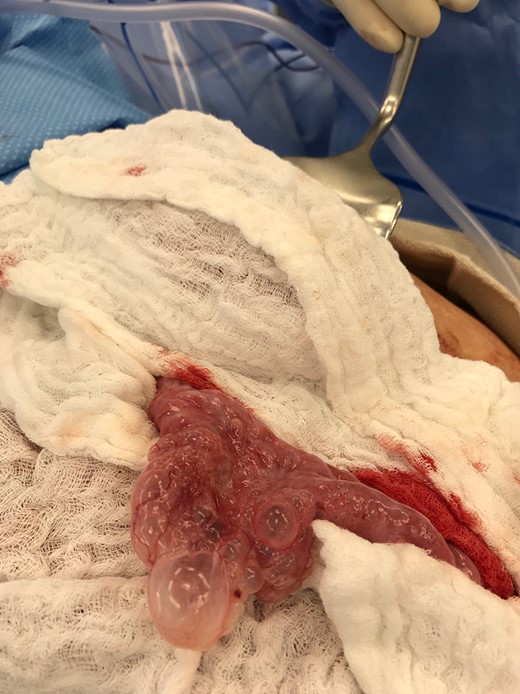
Multiple air filled cystic serosal nodules consistent with pneumatosis cystoides intestinalis.
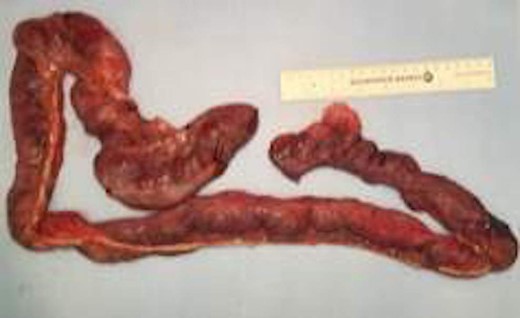
Multiple air filled cystic serosal nodules consistent with pneumatosis cystoides intestinalis.
On 7.8.18 the patient had return of bowel function complicated by blood per rectum and an episode of bloody emesis with drop in Hgb 9 > 7.0. The patient received 2 units PRBCs. GI consulted deemed blood per rectum likely from bleeding from anastomoses line. 7.8 Zosyn began for 7 days. Due to the concern for potential bleed, on 7.9 the patient had CTAP (Fig. 5) which demonstrated newly developed pneumatosis within ascending and transverse colon. Pt at this time had appropriate post-surgical abdominal pain, no leukocytosis 4.6, and was HDS. Decision was made to conservatively manage this form of colonic pneumatosis, with TPN, antibiotics, and bowel rest. 7.11 EGD revealed a 5 mm ulcer at duodenal bulb. CTAP (Fig. 6) on 7.14.18 demonstrated largely resolved pneumatosis of ascending and transverse colon. 7.17 Mechanical soft diet, discharged home.
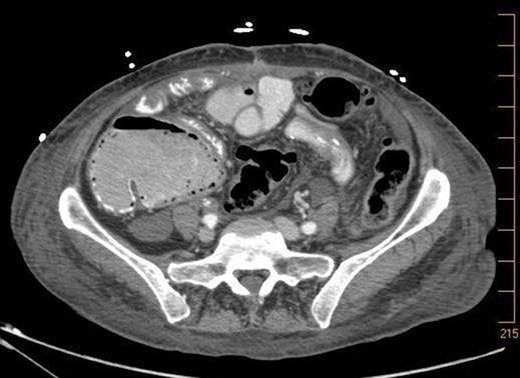
7.9.18: CTAP w/IV and PO contrast: pneumatosis within ascending and transverse colon, no evidence of bowel thickening or fat stranding, no evidence of bowel obstruction.
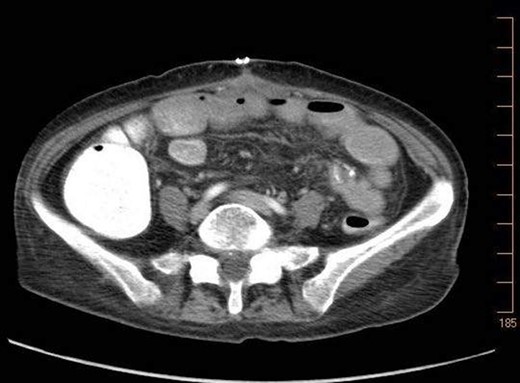
7.14.18: CTAP w/IV and PO contrast: largely resolved pneumatosis.
DISCUSSION/SUMMARY
Causes of PCI include the following: surgical anastomoses, obstruction, immunosuppression, ischemia from low flow states [4], lymphatic disorders [3, 8], all of which relate to our patient in this case report in terms of being risk factors. This patient had recent surgical interventions with primary anastomoses (2.2018), she was obstructed initially, she was immunosuppressed due to malnutrition, the patient had documented low flow states in which the patient’s hemoglobin was 7.0 on postoperative Day 4, and the patient had a known disorder of her intestinal lymphatics as documented in her surgical pathology of small intestinal serosal and mural cystic lymphangioma (Supplementary Fig. S7). This case report argues that her congenital lymphatic abnormality directly played a role in the development of her PCI. Fulminant PCI is associated with an acute bacterial process, sepsis, and necrosis of the bowel; while benign PCI is incidental and can be observed [5]. This article argues that lymphatic etiologies of PCI are benign and can be observed.
The role played by lymphatic abnormality in PCI is bolstered in one study of necrotizing enterocolitis in neonatal piglets where ligation of the lymphatic vessels in the submucosa, pneumatosis developed [5]. In another study, podoplanin, a mucoprotein preferentially expressed in lymphatic endothelial cells was found in the pericystic intestititum in 100% of cases (13 people), suggesting the states of PCI here were gas-distended lymphatics with the lymphatic epithelium ruptured [3].
Although, initially the PCI observed in this 57-year-old female patient was obstructive with concern or perforation; there is a question of whether this would have resolved conservatively without extirpation of jejunum. Therefore, attention to the hemodynamic stability of the patient, any evidence of clinical or laboratory ischemia should guide the management of PCI.
CONFLICT OF INTEREST STATEMENT
None declared.



