-
PDF
- Split View
-
Views
-
Cite
Cite
Piao Xu, Han Jiang, Fei Zheng, Qian Song, A case report of primary mucinous adenocarcinoma of the thymus and review of the literature, Journal of Surgical Case Reports, Volume 2025, Issue 7, July 2025, rjaf435, https://doi.org/10.1093/jscr/rjaf435
Close - Share Icon Share
Abstract
Thymic mucinous adenocarcinoma is an extremely rare and aggressive malignant tumor of the thymus with a very low 5-year survival rate. Here, we report a case of surgically resected primary mucinous adenocarcinoma of the thymus in a patient in his 60s who was admitted to the hospital with chest tightness for 2 weeks. The patient underwent median sternotomy and thymectomy, including resection of the brachiocephalic vein and partial resection of the superior vena cava wall. Histopathologic examination confirmed the diagnosis of mucinous adenocarcinoma, staged as pT2aN2M0 (IVB). Immunohistochemical analysis showed negative expression of CK20 and CK7, and preoperative carcinoembryonic antigen levels were within the normal range.
Introduction
Thymic adenocarcinomas have diverse histologic subtypes, including squamous cell carcinoma, basal cell carcinoma, mucoepidermoid carcinoma, adenocarcinoma, sarcomatoid carcinoma, clear cell carcinoma, lymphoepithelioid carcinoma, undifferentiated carcinoma, and other rare variants. Mucinous adenocarcinoma is a distinct subtype of thymic adenocarcinoma that was first officially recognized as thymic mucinous carcinoma in the World Health Organization Histological Classification of Thymic Tumors in 2004 [1]. Given that thymic mucinous carcinoma is extremely rare, with only about 20 cases reported in the literature to date, we present a case of surgically resected primary thymic mucinous carcinoma. The clinicopathological features and follow-up results of this rare case are comprehensively analyze in this report, which contributes to the limited understanding of primary thymic mucinous carcinoma.
Case presentation
A male in his 60s presented to our hospital with chest pain for a week. The patient had undergone coronary stenting in 2021. A computed tomography (CT) scan of the chest showed a round isointense mass in the anterior mediastinum measuring ~37 × 15 mm (Fig. 1A and B). Contrast-enhanced CT of the tumor showed mild homogeneous enhancement with punctate calcification (Fig. 1D). During surgery, the tumor was found to invade the left brachiocephalic vein and the lateral wall of the superior vena cava with evidence of nerve bundle involvement. Therefore, the patient underwent median sternotomy with total resection of the tumor (Fig. 1C), including the involved brachiocephalic vein, pericardium, and partially resected superior vena cava, and removal of peritumoral lymph nodes. Gross examination of the resection specimen revealed a tumor with a maximum diameter of 7 cm (Fig. 1F). The section was grayish-white and firm, and the internal mass measured 5.2 × 4 × 2 cm. Histopathologic examination under the microscope showed that the cancer cells were arranged in a glandular and wedge-shaped pattern, floating in pools of mucin. The cells exhibited marked atypia and showed infiltrative growth (Fig. 2A). Pathologic analysis of the margins of the specimen and the stump of the brachial vein showed no evidence of cancer cell involvement. Examination of six peritumoral lymph nodes revealed no metastatic cancer. Immunohistochemical staining results were as follows: CK (+), CK20 (−), CK7 (−), caudal-type homeobox transcription factor 2 (CDX-2) -2(+), villin (+), TTF-1 (−), P63 (−), S100 (−), CD5 (−), and Ki-67 of ~50% (magnification ×100) (Fig. 2). Post-operative pathological diagnosis confirmed mucinous adenocarcinoma. Pathological the patient received radiotherapy 1 month after surgery. Three months post-operatively, a follow-up chest CT performed in our hospital showed poorly structured layers with patchy areas of mild hypodensity in the operated area and no enhancement on contrast-enhanced scanning (Fig. 1E). The patient remains alive 6 months after surgery.
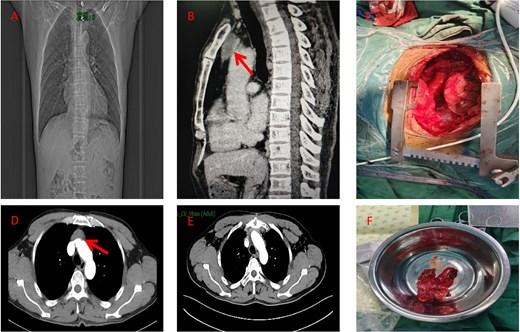
(A) Preoperative chest X-ray (coronal view). (B) Preoperative CT scan (sagittal view) showing the anterior mediastinal mass (arrow). (C) Intraoperative view of the resected specimen. (D) Preoperative CT scan (axial view) showing the anterior mediastinal mass (arrow). (E) Post-operative imaging at 3 months following surgery and radiotherapy (RT). (F) Photograph of the surgical specimen.
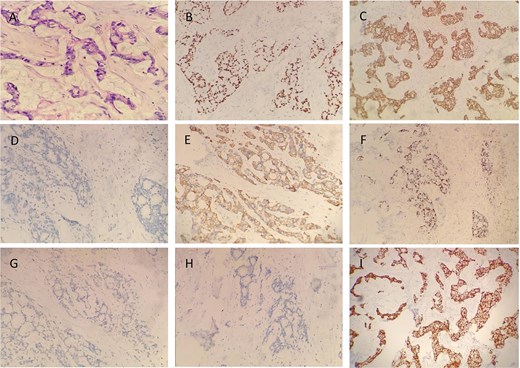
Immunohistochemical staining results revealed the following expression profile in the tumor cells: CK (+), CK20 (−), CK7 (−), CDX2 (+), villin (+), TTF-1 (−), P63 (−), S100 (−), CD5 (−), and Ki-67 ~50% (magnification ×100). Representative images of immunohistochemical staining are shown in figure (A) H&E, (B) CDX2, (C) CK, (D) CK7, (E) CK20, (F) Ki-67, (G) P63, (H) S100, and (I) villin (magnification ×100).
Discussion
Thymic adenocarcinoma is an extremely rare malignancy, and thymic mucinous adenocarcinoma is even rarer. Choi et al. reported the first case of primary thymic mucinous adenocarcinoma in 2003 [2]. In the previously documented cases, most patients were asymptomatic at presentation, while a small percentage presented with symptoms such as chest pain, shoulder pain, or chest tightness. Notably, patients with severe cases have been reported to be admitted to the hospital with angina pectoris due to compression of the coronary arteries by the tumor [3]. In this report, we present a unique case of a patient who had undergone coronary stenting 2 years earlier.
The differential diagnosis of mucinous adenocarcinoma of the thymus is a complex process. One of the main features of this type of cancer is the microscopic demonstration of mucin production. It is important to differentiate it from other tumors that may also produce mucin, such as mucoepidermoid carcinomas, teratomas, and metastatic mucinous adenocarcinomas from other primary sites. Mucoepidermoid carcinoma usually occurs in the salivary glands, especially the parotid gland, and frequently metastasizes to distant sites such as the lungs, liver, and bones. Teratomas, a type of germ cell tumor, may also contain mucus in their pathologic structure, but they are typically characterized by the presence of multiple tissue components. Metastatic mucinous adenocarcinomas originating from other sites such as the gastrointestinal tract or the lungs may also involve the thymus. In the present case, tumor cells were observed floating in pools of mucin, exhibiting marked cellular atypia. Preoperative screening showed that the tumor did not express alpha-fetoprotein or carcinoembryonic antigen (CEA). In addition, contrast-free cranial magnetic resonance imaging and color Doppler ultrasound of the liver, gallbladder, pancreas, spleen, and kidneys were performed, effectively ruling out the possibility of metastatic tumors from other sites.
In the present case, immunohistochemical analysis showed that the tumor cells expressed CK (+) and CDX-2 (+). To further elucidate the immunohistochemical features of thymic mucinous adenocarcinoma, we performed a retrospective analysis of previously reported cases. We systematically evaluated the positivity of CK7, CK20, CDX-2, CD5, v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT), transcriptional intermediary factor 1 (TIF-1), and CEA (Table 1). Of the 21 cases that underwent CK7 testing, 11 were positive (52%) and 4 were partially positive (19%). Of the 22 cases tested for CK20, 15 were positive (68%) and 2 were partially positive (9%). CDX-2 expression was evaluated in 18 cases, of which 15 were positive (83%) and 1 was partially positive (5%). CD5 was evaluated in 20 cases, of which 3 were positive (15%) and 12 were partially positive (60%). KIT and TIF-1 were tested in 8 and 19 cases, respectively, and all cases were negative. CEA, a broad-spectrum tumor marker commonly used in clinical practice, was positive in 10 out of 13 cases tested (77%). Based on our findings, we observed that CK7, CK20, CDX-2, and CEA showed relatively high positivity rates in previously reported cases of mucinous adenocarcinoma.CK20 is commonly expressed in the gastrointestinal tract and uroepithelium and is present in virtually all colorectal cancers, the majority of pancreatic cancers, and a significant proportion of gastric, biliary tract, and uroepithelial cancers [4, 5].CDX2 is a gut-specific gene regulator and is frequently detected in a variety of bowel cancers [6]. Immunohistochemical staining of CK20 and CDX2 helps to differentiate between primary thymic mucinous adenocarcinoma and metastatic gastrointestinal cancer [7, 8]. However, of the 22 reported cases of primary thymic mucinous adenocarcinoma, 20 cases (90%) showed positive or partially positive expression of the markers CK20 and CDX-2. It has been suggested that at least one of these markers is typically positive in cases of thymic mucinous adenocarcinoma [9]. This understanding aids in the further diagnosis of metastatic tumors of the gastrointestinal tract as well as thymic mucinous adenocarcinoma.
| Authors . | Year . | Sex . | Age . | Treatment . | Outcome . | Tumor size (cm) . | CK7 . | CK20 . | CDX-2 . | CEA . | CD5 . | KIT . | TTF-1 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al. | 2003 | M | 15 | S + RT | D/26 months | 8.0 × 5.0 × 4.0 | + | + | ND | ND | + | ND | ND |
| Seki Y et al. | 2004 | F | 34 | S + RT | D/14 months | 13.0 × 10.0 × 8.0 | ND | ND | ND | ND | ND | ND | ND |
| Takahashi et al. | 2005 | M | 59 | RT | D/11 months | not reported | +++ | − | − | ND | + | ND | − |
| Kapur et al. | 2006 | M | 41 | S + RT + CT | A/44 months | 10.5 × 8.0 × 2.5 | + | +++ | +++ | +++ | + | ND | − |
| Ra et al. (Case 1) | 2007 | F | 61 | S + RT | A/5 months | 5.2 × 3.9 | +++ | − | − | − | − | − | − |
| Ra et al. (Case 2) | 2007 | F | 82 | S | Dead of surgery complications | 14.5 × 7.0 | +++ | − | ND | − | + | − | − |
| Seki et al. | 2008 | M | 49 | S + RT + CT | A/11 months | 10 × 10 | +++ | +++ | ND | ND | − | ND | − |
| Maeda et al. (Case 1) | 2009 | F | 52 | S + RT + CT | A/11 months | 9.5 × 6.0 × 5.5 | − | +++ | +++ | +++ | +++ | ND | − |
| Maeda et al. (Case 2) | 2009 | M | 38 | S + RT + CT | D/12 months | 8.0 × 7.0 × 3.5 | +++ | +++ | +++ | +++ | + | ND | − |
| Maeda et al. (Case 3) | 2009 | M | 55 | S + RT + CT | D/24 months | 13.0 × 7.0 × 4.5 | − | +++ | +++ | +++ | + | ND | − |
| Seon et al. | 2012 | F | 66 | S | A/5 months | 11.0 × 9.5 × 9.0 | ND | +++ | ND | ND | +++ | ND | ND |
| Maghbool et al. | 2013 | F | 28 | S + RT + CT | A/30 months | not reported | + | +++ | +++ | ND | +++ | − | − |
| Moser et al. | 2015 | F | 39 | S | A/159 months | 6 | +++ | +++ | +++ | +++ | + | − | − |
| Sakanoue et al. | 2017 | F | 39 | S + CT | A/34 months | 6.5 | +++ | +++ | +++ | ND | + | ND | − |
| Kinoshita et al. | 2018 | F | 79 | S | A/14 months | 9.4 × 7.6 × 5.1 | − | +++ | +++ | ND | ND | ND | ND |
| Kalhor et al. (Case 1) | 2018 | M | 68 | S | A/10 months | 7.0 | +++ | +++ | +++ | +++ | + | − | − |
| Kalhor et al. (Case 2) | 2018 | M | 49 | S | A/12 months | 5.0 | +++ | +++ | +++ | +++ | + | − | − |
| Kalhor et al. (Case 3) | 2018 | M | 53 | S | Not reported | 6.0 | +++ | +++ | +++ | +++ | + | − | − |
| Himuro et al. | 2020 | F | 58 | S + RT | D/6 months | 7.5 × 2.8 × 5.2 | + | + | +++ | +++ | − | − | − |
| Tsukaguchi et al. | 2022 | F | 39 | S + RT + CT | D/5 months | 7.0 × 4.0 | − | +++ | +++ | ND | − | ND | − |
| Cho et al. | 2023 | M | 65 | CT | A/6 months | N/A | − | +++ | +++ | +++ | ND | ND | − |
| Zhang et al. | 2024 | M | 45 | S + RT + CT | A/49 months | 13.8 × 12.5 | +++ | − | + | ND | + | ND | − |
| Our case | M | 60 | S + RT | A/6 months | 5.2 × 4.0 × 2.0 | − | − | +++ | − | − | ND | − |
| Authors . | Year . | Sex . | Age . | Treatment . | Outcome . | Tumor size (cm) . | CK7 . | CK20 . | CDX-2 . | CEA . | CD5 . | KIT . | TTF-1 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al. | 2003 | M | 15 | S + RT | D/26 months | 8.0 × 5.0 × 4.0 | + | + | ND | ND | + | ND | ND |
| Seki Y et al. | 2004 | F | 34 | S + RT | D/14 months | 13.0 × 10.0 × 8.0 | ND | ND | ND | ND | ND | ND | ND |
| Takahashi et al. | 2005 | M | 59 | RT | D/11 months | not reported | +++ | − | − | ND | + | ND | − |
| Kapur et al. | 2006 | M | 41 | S + RT + CT | A/44 months | 10.5 × 8.0 × 2.5 | + | +++ | +++ | +++ | + | ND | − |
| Ra et al. (Case 1) | 2007 | F | 61 | S + RT | A/5 months | 5.2 × 3.9 | +++ | − | − | − | − | − | − |
| Ra et al. (Case 2) | 2007 | F | 82 | S | Dead of surgery complications | 14.5 × 7.0 | +++ | − | ND | − | + | − | − |
| Seki et al. | 2008 | M | 49 | S + RT + CT | A/11 months | 10 × 10 | +++ | +++ | ND | ND | − | ND | − |
| Maeda et al. (Case 1) | 2009 | F | 52 | S + RT + CT | A/11 months | 9.5 × 6.0 × 5.5 | − | +++ | +++ | +++ | +++ | ND | − |
| Maeda et al. (Case 2) | 2009 | M | 38 | S + RT + CT | D/12 months | 8.0 × 7.0 × 3.5 | +++ | +++ | +++ | +++ | + | ND | − |
| Maeda et al. (Case 3) | 2009 | M | 55 | S + RT + CT | D/24 months | 13.0 × 7.0 × 4.5 | − | +++ | +++ | +++ | + | ND | − |
| Seon et al. | 2012 | F | 66 | S | A/5 months | 11.0 × 9.5 × 9.0 | ND | +++ | ND | ND | +++ | ND | ND |
| Maghbool et al. | 2013 | F | 28 | S + RT + CT | A/30 months | not reported | + | +++ | +++ | ND | +++ | − | − |
| Moser et al. | 2015 | F | 39 | S | A/159 months | 6 | +++ | +++ | +++ | +++ | + | − | − |
| Sakanoue et al. | 2017 | F | 39 | S + CT | A/34 months | 6.5 | +++ | +++ | +++ | ND | + | ND | − |
| Kinoshita et al. | 2018 | F | 79 | S | A/14 months | 9.4 × 7.6 × 5.1 | − | +++ | +++ | ND | ND | ND | ND |
| Kalhor et al. (Case 1) | 2018 | M | 68 | S | A/10 months | 7.0 | +++ | +++ | +++ | +++ | + | − | − |
| Kalhor et al. (Case 2) | 2018 | M | 49 | S | A/12 months | 5.0 | +++ | +++ | +++ | +++ | + | − | − |
| Kalhor et al. (Case 3) | 2018 | M | 53 | S | Not reported | 6.0 | +++ | +++ | +++ | +++ | + | − | − |
| Himuro et al. | 2020 | F | 58 | S + RT | D/6 months | 7.5 × 2.8 × 5.2 | + | + | +++ | +++ | − | − | − |
| Tsukaguchi et al. | 2022 | F | 39 | S + RT + CT | D/5 months | 7.0 × 4.0 | − | +++ | +++ | ND | − | ND | − |
| Cho et al. | 2023 | M | 65 | CT | A/6 months | N/A | − | +++ | +++ | +++ | ND | ND | − |
| Zhang et al. | 2024 | M | 45 | S + RT + CT | A/49 months | 13.8 × 12.5 | +++ | − | + | ND | + | ND | − |
| Our case | M | 60 | S + RT | A/6 months | 5.2 × 4.0 × 2.0 | − | − | +++ | − | − | ND | − |
Abbreviations: CT, chemotherapy; RT, radiotherapy; S, surgery; D, died of disease; A, alive; F, female; M, male; ND, not done.
| Authors . | Year . | Sex . | Age . | Treatment . | Outcome . | Tumor size (cm) . | CK7 . | CK20 . | CDX-2 . | CEA . | CD5 . | KIT . | TTF-1 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al. | 2003 | M | 15 | S + RT | D/26 months | 8.0 × 5.0 × 4.0 | + | + | ND | ND | + | ND | ND |
| Seki Y et al. | 2004 | F | 34 | S + RT | D/14 months | 13.0 × 10.0 × 8.0 | ND | ND | ND | ND | ND | ND | ND |
| Takahashi et al. | 2005 | M | 59 | RT | D/11 months | not reported | +++ | − | − | ND | + | ND | − |
| Kapur et al. | 2006 | M | 41 | S + RT + CT | A/44 months | 10.5 × 8.0 × 2.5 | + | +++ | +++ | +++ | + | ND | − |
| Ra et al. (Case 1) | 2007 | F | 61 | S + RT | A/5 months | 5.2 × 3.9 | +++ | − | − | − | − | − | − |
| Ra et al. (Case 2) | 2007 | F | 82 | S | Dead of surgery complications | 14.5 × 7.0 | +++ | − | ND | − | + | − | − |
| Seki et al. | 2008 | M | 49 | S + RT + CT | A/11 months | 10 × 10 | +++ | +++ | ND | ND | − | ND | − |
| Maeda et al. (Case 1) | 2009 | F | 52 | S + RT + CT | A/11 months | 9.5 × 6.0 × 5.5 | − | +++ | +++ | +++ | +++ | ND | − |
| Maeda et al. (Case 2) | 2009 | M | 38 | S + RT + CT | D/12 months | 8.0 × 7.0 × 3.5 | +++ | +++ | +++ | +++ | + | ND | − |
| Maeda et al. (Case 3) | 2009 | M | 55 | S + RT + CT | D/24 months | 13.0 × 7.0 × 4.5 | − | +++ | +++ | +++ | + | ND | − |
| Seon et al. | 2012 | F | 66 | S | A/5 months | 11.0 × 9.5 × 9.0 | ND | +++ | ND | ND | +++ | ND | ND |
| Maghbool et al. | 2013 | F | 28 | S + RT + CT | A/30 months | not reported | + | +++ | +++ | ND | +++ | − | − |
| Moser et al. | 2015 | F | 39 | S | A/159 months | 6 | +++ | +++ | +++ | +++ | + | − | − |
| Sakanoue et al. | 2017 | F | 39 | S + CT | A/34 months | 6.5 | +++ | +++ | +++ | ND | + | ND | − |
| Kinoshita et al. | 2018 | F | 79 | S | A/14 months | 9.4 × 7.6 × 5.1 | − | +++ | +++ | ND | ND | ND | ND |
| Kalhor et al. (Case 1) | 2018 | M | 68 | S | A/10 months | 7.0 | +++ | +++ | +++ | +++ | + | − | − |
| Kalhor et al. (Case 2) | 2018 | M | 49 | S | A/12 months | 5.0 | +++ | +++ | +++ | +++ | + | − | − |
| Kalhor et al. (Case 3) | 2018 | M | 53 | S | Not reported | 6.0 | +++ | +++ | +++ | +++ | + | − | − |
| Himuro et al. | 2020 | F | 58 | S + RT | D/6 months | 7.5 × 2.8 × 5.2 | + | + | +++ | +++ | − | − | − |
| Tsukaguchi et al. | 2022 | F | 39 | S + RT + CT | D/5 months | 7.0 × 4.0 | − | +++ | +++ | ND | − | ND | − |
| Cho et al. | 2023 | M | 65 | CT | A/6 months | N/A | − | +++ | +++ | +++ | ND | ND | − |
| Zhang et al. | 2024 | M | 45 | S + RT + CT | A/49 months | 13.8 × 12.5 | +++ | − | + | ND | + | ND | − |
| Our case | M | 60 | S + RT | A/6 months | 5.2 × 4.0 × 2.0 | − | − | +++ | − | − | ND | − |
| Authors . | Year . | Sex . | Age . | Treatment . | Outcome . | Tumor size (cm) . | CK7 . | CK20 . | CDX-2 . | CEA . | CD5 . | KIT . | TTF-1 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al. | 2003 | M | 15 | S + RT | D/26 months | 8.0 × 5.0 × 4.0 | + | + | ND | ND | + | ND | ND |
| Seki Y et al. | 2004 | F | 34 | S + RT | D/14 months | 13.0 × 10.0 × 8.0 | ND | ND | ND | ND | ND | ND | ND |
| Takahashi et al. | 2005 | M | 59 | RT | D/11 months | not reported | +++ | − | − | ND | + | ND | − |
| Kapur et al. | 2006 | M | 41 | S + RT + CT | A/44 months | 10.5 × 8.0 × 2.5 | + | +++ | +++ | +++ | + | ND | − |
| Ra et al. (Case 1) | 2007 | F | 61 | S + RT | A/5 months | 5.2 × 3.9 | +++ | − | − | − | − | − | − |
| Ra et al. (Case 2) | 2007 | F | 82 | S | Dead of surgery complications | 14.5 × 7.0 | +++ | − | ND | − | + | − | − |
| Seki et al. | 2008 | M | 49 | S + RT + CT | A/11 months | 10 × 10 | +++ | +++ | ND | ND | − | ND | − |
| Maeda et al. (Case 1) | 2009 | F | 52 | S + RT + CT | A/11 months | 9.5 × 6.0 × 5.5 | − | +++ | +++ | +++ | +++ | ND | − |
| Maeda et al. (Case 2) | 2009 | M | 38 | S + RT + CT | D/12 months | 8.0 × 7.0 × 3.5 | +++ | +++ | +++ | +++ | + | ND | − |
| Maeda et al. (Case 3) | 2009 | M | 55 | S + RT + CT | D/24 months | 13.0 × 7.0 × 4.5 | − | +++ | +++ | +++ | + | ND | − |
| Seon et al. | 2012 | F | 66 | S | A/5 months | 11.0 × 9.5 × 9.0 | ND | +++ | ND | ND | +++ | ND | ND |
| Maghbool et al. | 2013 | F | 28 | S + RT + CT | A/30 months | not reported | + | +++ | +++ | ND | +++ | − | − |
| Moser et al. | 2015 | F | 39 | S | A/159 months | 6 | +++ | +++ | +++ | +++ | + | − | − |
| Sakanoue et al. | 2017 | F | 39 | S + CT | A/34 months | 6.5 | +++ | +++ | +++ | ND | + | ND | − |
| Kinoshita et al. | 2018 | F | 79 | S | A/14 months | 9.4 × 7.6 × 5.1 | − | +++ | +++ | ND | ND | ND | ND |
| Kalhor et al. (Case 1) | 2018 | M | 68 | S | A/10 months | 7.0 | +++ | +++ | +++ | +++ | + | − | − |
| Kalhor et al. (Case 2) | 2018 | M | 49 | S | A/12 months | 5.0 | +++ | +++ | +++ | +++ | + | − | − |
| Kalhor et al. (Case 3) | 2018 | M | 53 | S | Not reported | 6.0 | +++ | +++ | +++ | +++ | + | − | − |
| Himuro et al. | 2020 | F | 58 | S + RT | D/6 months | 7.5 × 2.8 × 5.2 | + | + | +++ | +++ | − | − | − |
| Tsukaguchi et al. | 2022 | F | 39 | S + RT + CT | D/5 months | 7.0 × 4.0 | − | +++ | +++ | ND | − | ND | − |
| Cho et al. | 2023 | M | 65 | CT | A/6 months | N/A | − | +++ | +++ | +++ | ND | ND | − |
| Zhang et al. | 2024 | M | 45 | S + RT + CT | A/49 months | 13.8 × 12.5 | +++ | − | + | ND | + | ND | − |
| Our case | M | 60 | S + RT | A/6 months | 5.2 × 4.0 × 2.0 | − | − | +++ | − | − | ND | − |
Abbreviations: CT, chemotherapy; RT, radiotherapy; S, surgery; D, died of disease; A, alive; F, female; M, male; ND, not done.
CD5 is a leukocyte marker expressed on differentiated thymocytes and found mainly on the surface of immune cells such as T lymphocytes. It is commonly used to distinguish thymic carcinomas from non-thymic malignancies and is an important marker for tumors of thymic origin [10–12]. However, a small percentage of cases, including the present case, do not express CD5. In addition, this case was also negative for TTF-1, a lung adenocarcinoma marker; the absence of TTF-1 further supports the diagnosis of primary thymic mucinous carcinoma rather than metastatic lung adenocarcinoma [13].
In the field of oncogene research in thymic mucinous adenocarcinoma, mutations, especially Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations, have been identified in three reported cases, whereas no mutations were found in the epidermal growth factor receptor (EGFR) or echinoderm microtubule-associated protein-like 4 (EML4)–anaplastic lymphoma kinase (ALK) fusion genes [14]. While KRAS mutations are usually associated with lung, colorectal, and pancreatic cancers, the identification of KRAS mutations in thymic mucinous adenocarcinomas is a new finding. The presence of mutations in the STK11/LKB1 gene was first described in a case report by Himuro et al. [15]. Similarly, a recent case report by Tsukaguchi et al. highlighted, for the first time, an association between thymic mucinous adenocarcinoma and mutations in the PIK3CA gene [16]. These findings have led to a better understanding of the pathogenesis of this rare malignancy and have provided important clues and directions for the development of targeted therapies.
In the treatment of thymic mucinous adenocarcinoma, complete surgical resection combined with adjuvant radiotherapy and chemotherapy is the mainstay of the current treatment strategy. This approach is necessary due to the high degree of malignancy and the fact that the tumors are usually large, with an average maximum diameter of 80 mm at the time of diagnosis [17]. In recent years, the use of targeted therapies and immune checkpoint inhibitors in thymic epithelial tumors has shown promise. These emerging therapeutic approaches may offer new options for the treatment of thymic mucinous adenocarcinoma [18], which, according to a review of the literature, the mean age of onset is 51 years (range: 15–82 years), with an almost equal gender distribution (11 females and 12 males). Of the 23 reported cases, 21 patients (91%) underwent surgical resection. However, post-operative survival was generally short, with a mean survival of 8 months due to the highly aggressive nature of the tumor. Distant metastases frequently develop after surgery, with common sites including the lung, bone, liver, cerebellum, and adrenal glands. Given the aggressive nature of this malignancy and the intraoperative finding of tumor invasion of the brachiocephalic vein and superior vena cava, we performed extended resection in this case and radiotherapy 1 month after surgery. After 6 months of follow-up, the patient remains free of recurrence.
Conclusion
This case was definitively diagnosed as primary thymic mucinous carcinoma. Based on the cases reported to date, there are no standardized treatment protocols or uniform immunohistochemical criteria for thymic mucinous adenocarcinoma. We hope to deepen our understanding of this rare malignancy through continued case reports and data accumulation. These efforts will contribute to the establishment of evidence-based guidelines for diagnosis, treatment, and prognostic evaluation, ultimately improving the clinical outcomes for patients with thymic mucinous carcinoma.
Author contributions
Piao Xu (Project management, data, Visualization, Conceptualization, Methods, Writing the manuscript), Han Jiang (Methods, Writing the manuscript), Fei Zheng (Data collection and Organization), Qian Song (Writing—review & editing). All the researchers have reviewed the article, reached a consensus, and agreed to submit it.
Conflict of interest statement
The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Jiangxi Provincial Natural Science Foundation (No: 20202ACBL206016).



