-
PDF
- Split View
-
Views
-
Cite
Cite
Paola De Angelis, Barbara Daniela Iacobelli, Filippo Torroni, Luigi Dall'Oglio, Pietro Bagolan, Fabio Fusaro, What else is hiding behind superior mesenteric artery syndrome?, Journal of Surgical Case Reports, Volume 2015, Issue 5, May 2015, rjv057, https://doi.org/10.1093/jscr/rjv057
Close - Share Icon Share
Abstract
The superior mesenteric artery syndrome (SMAS) is an uncommon condition in children. We describe a case of a 7-year-old boy with SMAS that occurred 3 years after a Deloyers' procedure for subtotal colonic Hirschsprung who was admitted for bilious vomit, abdominal pain and diarrhea due to unrecognized celiac disease. This case emphasize that SMAS in children needs a close medical and surgical follow-up to avoid an underestimation of early clinical signs unrelated to surgery.
INTRODUCTION
The superior mesenteric artery syndrome (SMAS) is an uncommon condition in children resulting from vascular compression of the third part of the duodenum between the superior mesenteric artery (SMA) and the aorta. We describe a case of a 7-year-old boy with SMAS that occurred 3 years after a Deloyers’ procedure for subtotal colonic Hirschsprung.
CASE REPORT
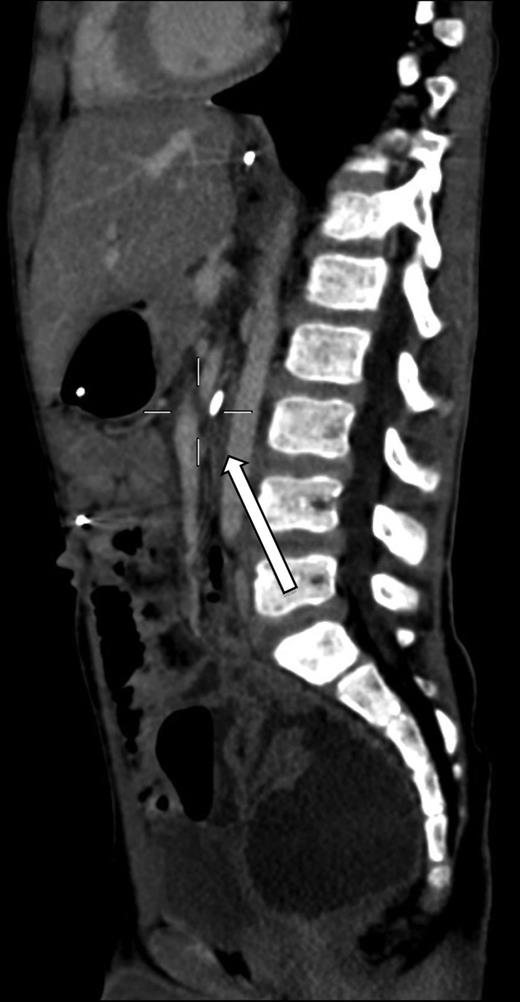
A 7-year-old boy who had been treated at 3 years of age for a subtotal colonic Hirschsprung disease with a Deloyers’ procedure was admitted for persistent bilious vomit associated with recurrent episodes of postprandial abdominal pain and diarrhea. The parents reported a progressive weight loss of 3.5 kg during the last 3 years. The patient was emaciated with severe chronic malnutrition; the weight and the length were 15.19 kg and 113 cm [body mass index (BMI): 11.9 kg/m2], both between the third and the fifth percentile, respectively. The clinical abdominal examination was normal. A plain abdominal X-ray showed a distended stomach with paucity of gas in the distal bowel. An abdominal ultrasound confirmed a dilated duodenum without signs of mechanical obstruction of the small bowel and a normal relationship between the superior mesenteric vessels. An upper gastrointestinal contrast study confirmed dilatation of the first and second portions of the duodenum with little progression of contrast medium across the third portion released in left lateral position (Fig. 1). An SMAS was confirmed by a computed tomography (CT) scan that showed a reduction in retroperitoneal preduodenal and mesenteric fat and compression of the third duodenum by the superior mesenteric artery with an acute 13° angle and a 7.5 mm distance from the aorta (Fig. 2). A gastrointestinal endoscopy confirmed the dilatation of the duodenum with an inflammatory mucosa and a normal jejunum. Gastric and duodenal biopsies were performed and a nasojejunal tube was guided through the duodenum to the Treitz to start enteral feeding (Fig. 3).
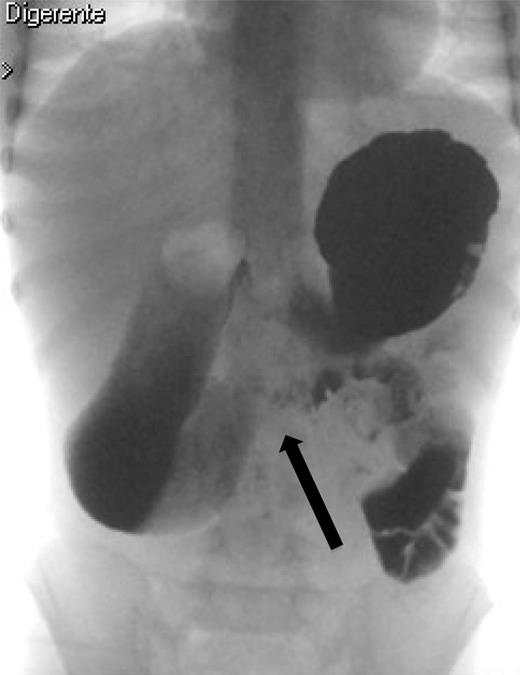
The upper gastrointestinal contrast study shows poor progression of contrast medium across duodenal obstruction. Arrow shows obstruction of the third duodenum.
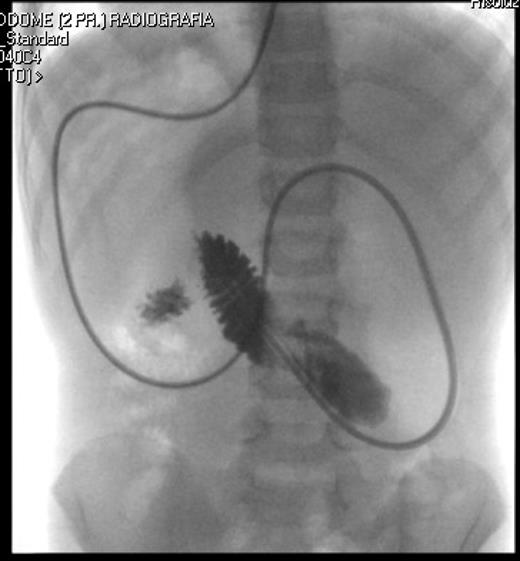
Correct position of the nasojejunal tube after endoscopic placement.
The duodenal biopsies showed a villous atrophy, crypt hyperplasia and increased intraepithelial lymphocytosis compatible with a celiac disease that was confirmed by an increased serum level of immunoglobulin (Ig)A anti-tissue transglutaminase (TG2; 270 U/ml; normal value <4 U/ml), and the human leucocyte antigen (HLA)-DQ2 genotype at the analysis of HLA Class II region. After 3 weeks, the patient gained 2 kg tolerating a gluten-free diet in addition to his tube feeding, and he was discharged to go home. At the scheduled 4-week outpatient control, he had gained 1.6 kg, the enteral feeding tube was removed and he was allowed to eat a full gluten-free diet. At the 1-year follow-up, the nutritional status had improved significantly with a BMI gain from 11.9 kg/m2 (<5th percentile) to 16 kg/m2 (56th percentile).
DISCUSSION
The SMAS results from vascular compression of the third part of the duodenum due to the reduction in the acute angle and distance between the superior mesenteric artery and the abdominal aorta. Several predisposing conditions for SMAS have been identified, including abdominal surgical procedures and dietary disorders with a significant weight loss [1–3]. In our patient, the etiology of SMAS may be related to two causes: the Deloyers' procedure employed for the treatment of total Hirschsprung disease, and undiagnosed celiac disease with associated malabsorption and weight loss. The Deloyers’ procedure allows to obtain a colorectal or coloanal anastomosis. After a left colectomy extended to the transverse colon and rectum, the right colon is rotated in a craniocaudal and a counter clockwise direction to avoid compression of the terminal ileum, and it is pulled down into the pelvis. Similar to surgical procedures of the ileoanal pouch performed in adult patients, the Deloyers' maneuver may cause traction in small bowel mesentery changing the relationship between the vascular structures forming the aortomesenteric angle [4]. This anatomical modification may have reduced the aortomesenteric angle making our patient susceptible to SMAS [5, 6]. The progressive weight loss related to malabsorption associated with celiac disease may have led to depletion of the retroperitoneal preduodenal and mesenteric fat with a further decrease of the aortomesenteric angle and distance, worsening the duodenal obstruction. SMAS diagnosis was derived from the upper gastrointestinal radiography associated with the CT scan that demonstrated the location of the duodenal obstruction between the aorta and the superior mesenteric artery with an acute aortomesenteric angle and a reduced aortomesenteric distance measuring 13° and 7.5 mm, respectively. In the adult population, the aortomesenteric angle and distance range from 20° to 70° and from 10 to 34 mm, respectively [7]. The normal value in children is unknown. Upper gastrointestinal endoscopy, as used in this patient, may be a useful diagnostic and therapeutic tool to rule out associated pathologies that may create a predisposition for the development of SMAS, and the placement of a jejunal tube to bypass the obstruction is useful to administer hypercaloric enteral feeding avoiding parenteral nutrition [8]. In conclusion, as reported in adults after ileoanal pouch anastomosis, SMAS should be considered as the cause of upper gastrointestinal obstruction in children treated by a Deloyers' procedure or ileoanal anastomosis. Close attention must be given during preparation of the mesentery avoiding any possible source of traction and employing, if necessary, the ruses for mesenteric lengthening in adult patients [9]. It is always important to follow the clinical course of operated children for they can present symptoms and signs not related to surgery as in this reported case. According to the most recent guidelines by ESPGHAN on celiac disease, our patient was able to meet the criteria outlined to avoid duodenal biopsy with serum levels of IgA anti-TG2 >10 falling within the normal value with a strong suspected clinical picture originally interpreted as a sequela of the previous surgery [10]. In this patient, we were forced to take a journey back from duodenal biopsies to serology IgA anti-TG2 when the clinical findings already led to diagnosis. This process that led to severe malnutrition with worsening of SMAS, to several radiological and invasive procedures and to several hospitalizations could have been avoided. Only a close medical and surgical follow-up can avoid an underestimation of early clinical signs unrelated to surgery.
CONFLICT OF INTEREST STATEMENT
None declared.



