-
PDF
- Split View
-
Views
-
Cite
Cite
Adarsh Vijay, Lakshmi Ram, Renol Koshy Mathew, Muhammad Zafar Chawdhery, Solid pseudopapillary tumor of the pancreas in a patient with cervical cancer: relation of E-cadherin/β-catenin adhesion complex in their carcinogenesis, Journal of Surgical Case Reports, Volume 2015, Issue 4, April 2015, rjv034, https://doi.org/10.1093/jscr/rjv034
Close - Share Icon Share
Abstract
Solid pseudopapillary tumor (SPT) of the pancreas is one of the most uncommon histotypes of all exocrine pancreatic neoplasms. Disorganization of E-cadherin and β-catenin mutations, two key components of the Wnt signal transduction pathway, has been implicated in the development of SPT, but not other pancreatic tumors. Loss of E-cadherin/β-catenin proteins and tyrosine phosphorylation of E-cadherin/β-catenin have been postulated in cervical carcinogenesis and cancer invasion. A 38-year-old married woman, who had undergone brachytherapy, radiotherapy and chemotherapy for cervical cancer in Philippines in 2011, was admitted to our hospital after follow-up CT scan of abdomen in 2012 revealed a lesion in the tail of pancreas. The patient underwent distal pancreatectomy and splenectomy. The pathological diagnosis was SPT of pancreas. We suspect that the concurrent SPT pancreas and cervical cancer in this woman were triggered by a primary insult, a process in which E-cadherin/β-catenin/Wnt-signaling pathway played important roles.
INTRODUCTION
Solid pseudopapillary tumor (SPT) of the pancreas are one of the most uncommon histotypes (<3%) of all exocrine pancreatic neoplasms [1]. The cell origin of SPT and tumorigenesis are still enigmatic and warrants more research. Very rarely have they been associated with pancreatic or extra pancreatic anomalies. Here we present a case of pancreatic SPT with concomitant cervical cancer and postulate the role of E-cadherin/catenin adhesion complex in their carcinogenesis.
CASE REPORT
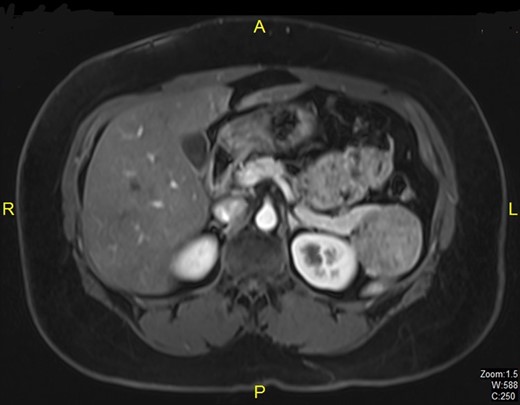
A 38-year-old married woman, who had undergone brachytherapy, radiotherapy and chemotherapy for cervical cancer in the Philippines in the year 2011, was admitted to our hospital after a follow-up CT scan of the abdomen in 2012 revealed a lesion in the tail of pancreas which on subsequent MRI scan was diagnosed as benign intraductal papillary mucinous neoplasm measuring 5.1 × 4.6 cm (Fig. 1). Physical examination revealed no abnormal findings. Tumor markers including CA 19-9 and CA 125 were within normal limits. Cervical smear was negative for malignancy. The patient underwent distal pancreatectomy and splenectomy. Histopathological examination showed solid pseudopapillary tumor of the pancreas (Fig. 2). Postoperatively she developed unilateral lower lobe consolidation. She recovered smoothly and was discharged 8 days after resection.
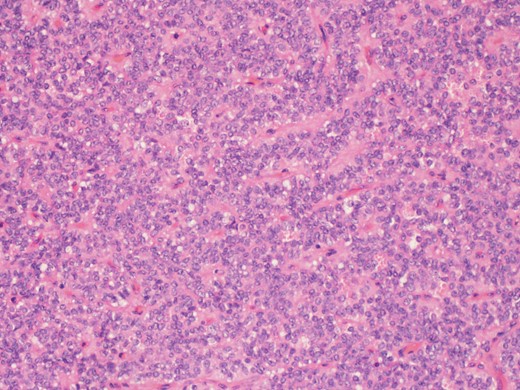
A higher power picture shows the pseudopapillae are lined by layers of bland cells and the nuclei are oval with finely stippled chromatin, nuclear grooves and indistinct nucleoli (H&E ×60).
DISCUSSION
SPT of the pancreas was first described by Virginia Frantz in 1959 [2]. In the past, this entity has been described by different names in the literature such as solid cystic tumor, papillary cystic neoplasm, papillary epithelial neoplasm, solid cystic acinar cell tumors and Frantz's tumor. In 1996 the World Health Organization (WHO) finally termed this neoplasm as ‘solid pseudopapillary tumor’ of the pancreas.
There exists no consensus on a preferable location of the tumor within the pancreas. They usually affect young women at an average age of 28 years with a female:male ratio of 10:1 [3]. However, sporadic rare cases in men and in the elderly have also been reported. The tumor has a low-grade malignant potential and tends to have a favorable prognosis after resection, even in the presence of metastatic disease (up to 20% of cases) [3]. The overall 5-year survival exceeds 90% including patients with metastatic disease [4]. Males, elderly, histopathologically atypical tumors and incomplete resections have been linked to a higher risk of recurrence and death [4].
Primitive pancreatic cells (ductal/epithelial/endocrine) and female genital bud cell lines have been linked to the origin of these tumors [5]. These tumors are found to express progesterone receptors but not estrogen receptors. Generally, there is no specific clinical syndrome associated with SPT of the pancreas. However, SPT has been reported to occur occasionally in patients with congenital pancreatic anomalies (pancreatic dorsal agenesis, pancreatic divicum), congenital urogenital anormalies (solitary kidney and uterus didelphys) and congenital mental anomaly (Mulvihill-Smith syndrome) [6]. Here we present a case of pancreatic SPT with concomitant cervical cancer.
Disorganization of E-cadherin and β-catenin mutations, two key components of the Wnt signal transduction pathway have been implicated in the development of SPT, but not other pancreatic tumors [7]. SPT almost consistently harbors β-catenin gene (CTNNB1) mutations in exon 3 [7], E-cadherin and β-catenin are involved in cellular differentiation and growth. Reduced E-cadherin expression was shown to promote epithelial cell invasiveness, dedifferentiation and metastases in various human carcinomas including cervical cancer [8]. Loss of E-cadherin/catenin proteins and the tyrosine phosphorylation of E-cadherin/catenin have been postulated in cervical carcinogenesis and cancer invasion [8]. Thus we are able to identify abnormalities in expression of E-cadherin/catenin complex in both SPT tumorigenesis and cervical carcinogenesis.
Similar concomitant tumors have not been reported to date. We suspect that the concurrent SPT pancreas and cervical cancer in this woman were triggered by a primary insult, a process in which E-cadherin/ β-catenin/ Wnt-signaling pathway played important roles.
In conclusion, solid pseudopapillary tumors of the pancreas are rare and are not known to be related to any clinical syndrome. An association with cervical cancer is unheard of. Further research on the underlying molecular genetic mechanism may enable establish a relationship between SPT of pancreas and cervical cancer.
FUNDING
Publication charges funded by Medical Research Center, Hamad Medical Corporation.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- radiation therapy
- computed tomography
- signal transduction
- brachytherapy
- mutation
- cancer
- chemotherapy regimen
- cervical cancer
- adhesions
- follow-up
- marriage, life event
- pancreatic neoplasms
- philippines
- phosphorylation
- splenectomy
- abdomen
- diagnosis
- neoplasms
- pancreas
- tyrosine
- carcinogenesis
- e-cadherin
- signal transduction pathways
- pancreatectomy, distal
- pancreas tail
- solid pseudopapillary tumor of the pancreas



