-
PDF
- Split View
-
Views
-
Cite
Cite
Mohammed A. Sbeih, Ryan Engdahl, Marina Landa, Oreoluwa Ojutiku, Norman Morrison, Hector Depaz, A giant phyllodes tumor causing ulceration and severe breast disfigurement: case report and review of giant phyllodes, Journal of Surgical Case Reports, Volume 2015, Issue 12, December 2015, rjv162, https://doi.org/10.1093/jscr/rjv162
Close - Share Icon Share
Abstract
Phyllodes tumors are rare fibroepithelial tumors that account for <1% of the breast tumors in women. These tumors are often benign unilateral lesions of the female breast (70%). Less common are malignant phyllodes, which have the potential for hematogenous spread. Phyllodes tumors can be seen in all age groups, and the median age of presentation is 45 years. Surgery is the main form of treatment. Wide excisions with margins of 1cm are suggested. While smaller and moderate size phyllodes may typically be seen, gigantic ones are very rare. These may be seen in neglected tumors. By definition, a giant phyllodes tumor is one larger than 10 cm in diameter. We report a gigantic phyllodes tumor that grew over 7 years period causing significant ulceration and disfigurement and review features of these tumors and management.
INTRODUCTION
Phyllodes tumors are rare fibroepithelial tumors that account for <1% of the breast tumors. These tumors are often benign. A giant breast mass in females in the fourth and fifth decades of life should prompt consideration of phyllodes. Currently, biopsy is needed to diagnose phyllodes. The treatment for phyllodes tumors is wide local excision with sufficient margin of normal breast tissue or mastectomy. Mastectomy is often required in the case of giant phyllodes. Appropriate cutoff values for tumor size and associated prognosis are not well defined.
CASE REPORT
A 41-year-old Asian female presented to the outpatient breast surgery clinic in our hospital with a 7-year history of an enlarging left breast mass. Over the last 8 months, she reported discomfort as well as ulceration with occasional bleeding. The worsening symptoms prompted her to seek medical care. On further review, she stated that she had sought no help as she thought the mass might disappear spontaneously. She was otherwise healthy without prior surgery. She had no family history of breast or ovarian cancer. On physical examination, she had a large nodular mass measuring ∼25 × 20 cm involving the entire left breast with two periareolar areas of skin ulceration measuring ∼2 × 2 and 3 × 4 cm (Fig. 1). There was a palpable left axillary lymph node 2 cm in diameter. Her right breast and the rest of her clinical examination were normal.
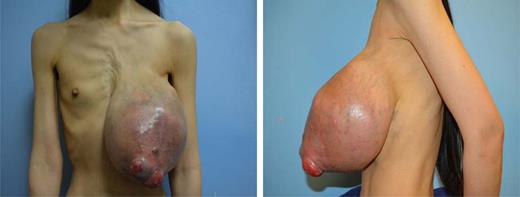
The left breast mass measured 25 × 20 cm with skin ulceration and severe disfigurement. The contralateral breast was normal.
Due to pain and size of the right breast mass, the patient was unable to have mammography. Bilateral breast and axillary ultrasonography was performed. This showed a large heterogeneous solid mass with internal vascularity replacing all normal expected left breast tissue (Fig. 2). The size was difficult to be accurately defined on ultrasonography due to its large size. In the left axilla, a 1.8-cm nonspecific lymph node was identified with slightly prominent cortices. There were no suspicious findings in the right breast or right axilla. The patient had core biopsies of the left breast and left axillary node. Initial histopathological analysis suggested a fascicular pseudoangiomatous stromal hyperplasia or phyllodes tumor. The left axillary lymph node biopsy revealed chronic lymphadenitis without neoplastic cells. Further workup with a chest and abdomen computed tomography (CT) scan was performed, which showed no evidence of metastatic lesion.
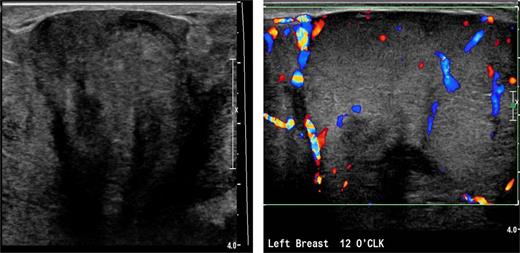
Left breast ultrasound demonstrates a heterogeneous solid mass with internal vascularity replacing the normal breast tissue.
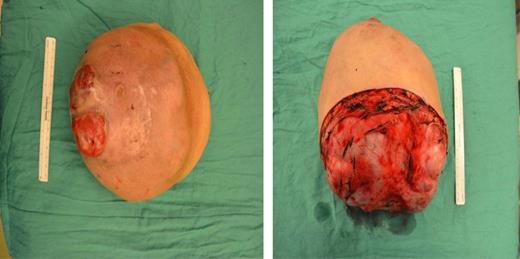
A left mastectomy was performed. The excised mass was 20 × 20 × 25 cm in size (Fig. 3). The tumor did not appear grossly to be invading the chest wall. The pectoralis fascia was free of tumor invasion (Fig. 4). The left chest wall defect was covered temporarily with dual layers dermal regeneration template consists of thin outer layer of silicone and thick inner matrix layer of pure bovine collagen and glycosaminoglycan (INTEGRA®) in the same operation, and subsequently replaced with split thickness skin graft 5 weeks later (Fig. 5). Final histological examination was consistent with phyllodes tumor with low-grade malignant features with negative margins (Fig. 6).
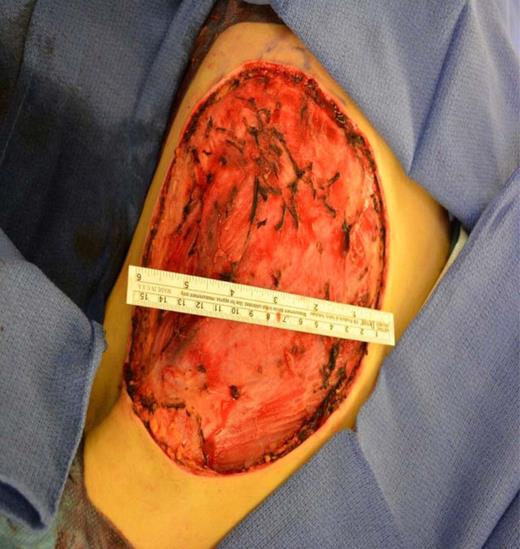
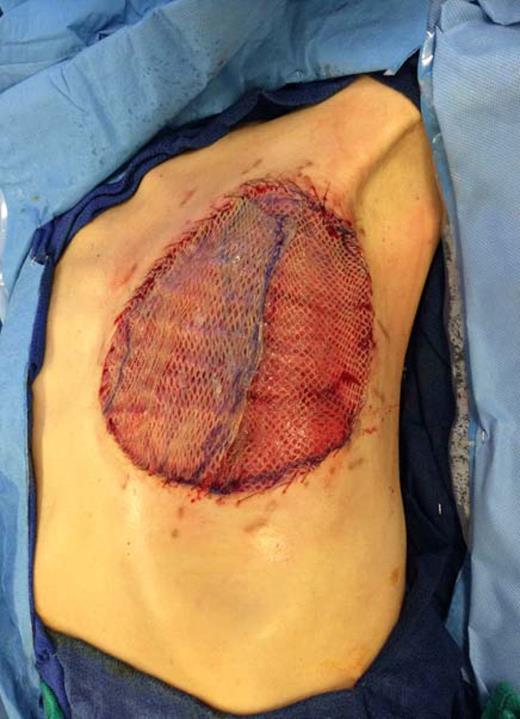
Coverage of the left chest wall defect with a split thickness skin graft.
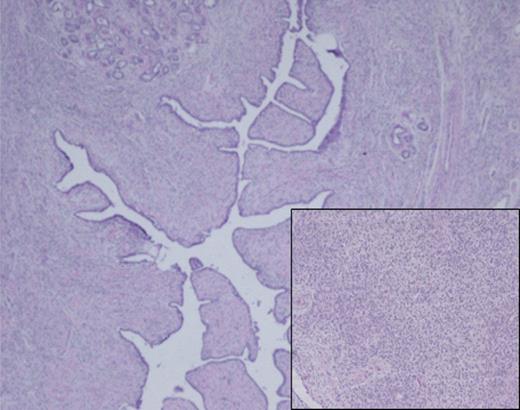
Histological analysis of the surgical specimen demonstrated a diffuse fibroepithelial lesion showing an intracanalicular growth pattern. Lobular architecture is preserved for the most part, and a variably cellular stromal component was seen throughout with focal stromal overgrowth pattern. Densely cellular spindle cell component was also noted (inset).
DISCUSSION
Phyllodes tumors are rare fibroepithelial breast tumors that have both epithelial and stromal elements. These tumors comprise <1% of primary breast tumors [1]. They typically present as mobile rounded nodules or masses, usually painless, and may have rapid growth. The majority are clinically benign [2]. They are usually unilateral with <1% of cases occurring bilaterally [3, 4]. The etiology of phyllodes tumors remains unknown. Although, some suggest hyperestrinism or breast trauma may be a predisposing factors [5, 6]. Though imaging may aid in the diagnosis in some cases, mammography is often nondiagnostic. Reports using ultrasonography characterize a phyllodes as a solid lobulated nodule of well-defined contours with heterogeneous echotexture, which may be associated with cystic components [7]. As in our case, larger tumors often are characterized by imaging that includes CT scans and evaluation for metastasis. Biopsy remains the mainstay in establishing the diagnosis.
The World Health Organization (WHO) classification of phyllodes tumors includes three broad categories (benign, borderline and malignant). This classification does not include the size of the tumor. The WHO classification was made according to the mitotic activity observed, degree of cellular atypia, characteristics of tumor margins and the presence of stromal growth. The benign variants in general do not metastasize. However, they may grow aggressively and can recur locally. The malignant variants of phyllodes can metastasize hematogenously most commonly to the lungs, but it can metastasize to the bone, heart and liver as well. There is a continuing debate over the prognostic significance of tumor size. Thus, appropriate cut-off values for tumor size and associated prognosis have not been well defined.
The treatment for phyllodes tumors is wide local excision with sufficient margin of normal breast tissues irrespective of the histological features (at least 1 cm negative margin) [8]. Some tailoring of this approach is applied to clearly malignant tumors to obtain negative margins. Axillary lymph nodes are excised only if clinically enlarged or palpable, as phyllodes tumors do not typically metastasize via the lymphatics. Some authorities recommend mastectomy for borderline or malignant phyllodes tumors, especially if free margin cannot be achieved, or in cases of local tumor recurrence. There is currently no evidence of proven benefit of adjuvant therapies, but they can be considered for high-risk phyllodes tumor, including those >5 cm, or with stromal overgrowth, or >10 mitoses per high-power field, or for those tumors with positive margins [9]. However, some have suggested that the risk of local recurrence or metastasis is <25% of tumors with a ‘malignant’ histologic grade [10]. Because there is a risk of local recurrence and for some tumors a risk of metastatic disease, long-term follow-up is mandatory. In the future, molecular analysis of tumor tissue may play a role in defining the treatment for phyllodes.
CONFLICT OF INTEREST STATEMENT
None declared.



