-
PDF
- Split View
-
Views
-
Cite
Cite
Vid Fikfak, Puja Gaur, Min P. Kim, Endoscopic management of Boerhaave's syndrome presenting with hematemesis, Journal of Surgical Case Reports, Volume 2014, Issue 11, November 2014, rju110, https://doi.org/10.1093/jscr/rju110
Close - Share Icon Share
Abstract
Hematemesis is an uncommon yet challenging presentation of Boerhaave's syndrome. Here, we present minimally invasive management of an esophageal perforation with hematemesis using esophageal stenting in an elderly male with multiple comorbidities.
INTRODUCTION
Boerhaave's syndrome is an uncommon, but highly morbid cause of esophageal perforation, which classically presents with repeated episodes of forceful retching and vomiting, followed by epigastric pain [1]. When presenting with hematemesis, the diagnosis of Boerhaave's syndrome is often missed and inappropriately treated. Here, we present the workup and successful minimally invasive treatment of a patient who presented with hematemesis and was found to have Boerhaave's syndrome.
CASE REPORT
An 81-year-old male presented to the emergency department with hematemesis and epigastric pain. On the evening of presentation, the patient reported difficulty in swallowing a piece of meat during dinner, which led to several episodes of retching followed by bloody emesis. His medical history was significant for extensive cardiovascular diseases including hypertension, stroke and pulmonary embolism for which an inferior vena cava filter had been in place. He had also undergone a carotid endarterectomy and taking daily aspirin. On initial presentation, the patient was afebrile, in sinus rhythm and normotensive. He had several episodes of hematemesis in the emergency department, and a nasogastric tube was placed with 500 ml of sanguineous output. Chest examination did not reveal any subcutaneous air, and lungs were clear bilaterally. On abdominal exam, however, the patient had tenderness to deep palpation of the epigastrium without any signs of peritonitis. Laboratories were significant for leukocytosis to 16.5 × 109/l and hemoglobin of 11.6 × 109/l. A computed tomography (CT) scan of the abdomen and pelvis with oral contrast was performed, which showed extraluminal contrast extending around the esophagus and around the left crus of the diaphragm that was compatible with esophageal perforation (Fig. 1A). The patient was resuscitated with fluids, started on broad spectrum antibiotics and after reviewing the CT scan was taken to the operating room for endoscopy, which revealed a 7-cm long esophageal perforation, starting at 29 cm and covered with blood clot. The gastroesophageal junction was identified at 42 cm from the incisors. About 1 l of blood was suctioned from the stomach and the GE junction was again visualized in retrograde view for possible involvement. There was no evidence of a tear in the gastric wall and no active bleeding was visualized. A partially covered self-expanding metal WallFlex stent (Boston Scientific) was introduced over a wire into the esophagus and deployed, covering the entire length of the perforation (Fig. 1B). The stent was bridled in place with an umbilical tape and we proceeded with an exploratory laparoscopy, which did not reveal any evidence of purulence near the area of the hiatus. A laparoscopic feeding jejunostomy tube was also placed at the time. Postoperative course was uneventful. The patient was kept nil per os for the next month, on tube feeds and antibiotics. His leukocytosis resolved over the ensuing days and his hemoglobin remained stable. A month later he presented to the hospital for esophageal stent removal. After endoscopically removing the stent, small healing ulcerations were identified in the area of previous perforation (Fig. 2A and B). An esophagram was performed using water soluble Omnipaque contrast to evaluate for a possible leak at the ulceration sites; however, no frank leak was found (Fig. 2C). The patient was then started on a clear liquid diet and discharged home. He was slowly advanced to a regular diet over the next several weeks which he tolerated well without any further complications.
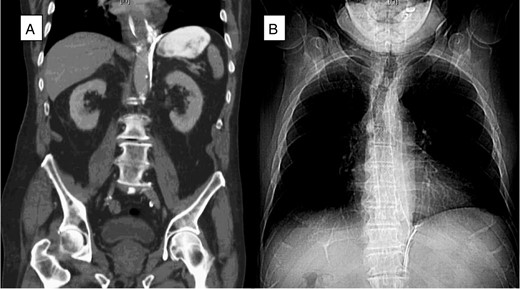
(A) A CT scan of the abdomen (coronal plane) demonstrated extraluminal contrast extending around the esophagus and around the left crus of the diaphragm. (B) Chest X-ray after esophagogastroduodenoscopy (EGD) and esophageal stent placement with stent in good position.
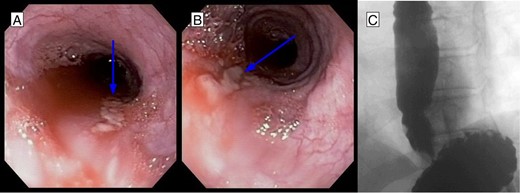
(A, B) EGD after stent removal revealed small healing lacerations in the area of previous perforation. (C) Follow-up esophagram without evidence of leak of contrast.
DISCUSSION
Boerhaave's syndrome is a rare, but morbid, condition with an associated 20–40% mortality [1, 2]. The diagnosis is often difficult as disease history and classic signs of forceful retching and vomiting followed by epigastric and retrosternal chest pain may be absent in over 20% of patients [3, 4]. Presentation with hematemesis is extremely rare and may lead to inappropriate workup for Mallory–Weiss syndrome and delayed treatment of underlying esophageal perforation. Once identified with imaging, the condition is managed either surgically, endoscopically with placement of a coated esophageal stent or conservatively based on the patient's condition, comorbidities, presence of sepsis and time from onset of first symptoms. Due to a broad spectrum of presentations and multiple treatment options, several treatment algorithms have been proposed, which recognize the utility of esophageal stenting for early non-septic patients, and although this treatment modality has been widely acknowledged, a recent retrospective study evaluating morbidity and mortality of early endoscopy and stent placement in patients with Boerhaave's syndrome has shown increased mortality when compared with early operative intervention [1, 5]. Multiple factors have been shown to influence the outcomes of stenting, including the ability to cover the entire length of the esophageal perforation which is often a challenge with most perforations presenting in the distal esophagus or the esophagogastric junction. Our aim was to demonstrate successful use of stenting in the setting of a bleeding perforation involving the mid-esophagus in a patient who was a poor surgical candidate due to multiple comorbidities. We recommend individualizing such endoscopic management of esophageal perforations to select cases based on the comorbidities of the patient and the amount of tissue loss from the injury.
ACKNOWLEDGEMENT
This research was supported by Thoracic Surgery Fund from Houston Methodist Foundation.



