-
PDF
- Split View
-
Views
-
Cite
Cite
Bonnie Wang, Seong-Jin Moon, William C. Olivero, Huan Wang, Cortical blindness as a rare presentation of cerebral venous thrombosis, Journal of Surgical Case Reports, Volume 2013, Issue 5, May 2013, rjt035, https://doi.org/10.1093/jscr/rjt035
Close - Share Icon Share
Abstract
Cerebral venous thrombosis (CVT) remains a diagnostic and therapeutic challenge for clinicians. Manifesting in a remarkably wide spectrum of symptoms and signs, CVT often presents in a misleading fashion—if unrecognized or misdiagnosed, it carries potentially fatal consequences. Visual loss is quite rare as the initial presentation of CVT and is typically a finding more frequent in chronic cases with associated papilledema on funduscopy Ferro, Lopes, Rosas and Fontes (Delay in Hospital Admission of Patients with Cerebral Vein and Dural Sinus Thrombosis. Cerebrovasc Dis 2005;19:152–6). We report a rare case of acute cortical blindness as the initial presentation of CVT in an 18-year-old female patient and review the current literature.
INTRODUCTION
Cerebral venous thrombosis (CVT) remains a diagnostic and therapeutic challenge for clinicians. Visual loss is quite rare as the initial presentation of CVT and is typically a finding more frequent in chronic cases with associated papilledema on funduscopy [1]. We report a case of acute cortical blindness as the initial presentation of CVT in an 18-year-old female patient.
CASE REPORT
An 18-year-old female with a three-week history of headaches presented with an acute onset of blindness in both eyes after her afternoon nap. Physical examination demonstrated normal pupils and funduscopic exam, but without light perception in either eye. Cranial nerve examinations were otherwise unremarkable. Speech was intact with no naming or repetition deficits. Although alert, she was mildly confused. No isolated motor or sensory deficits were identified in the extremities. Deep tendon reflexes were 3+ in the upper extremities and 4+ in the lower extremities. No ankle clonus was noted, and plantar reflexes were equivocal.
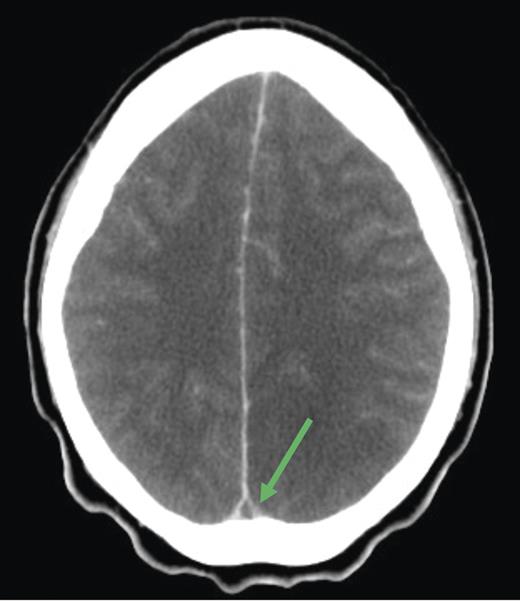
Cranial computed tomography (CT) demonstrated subtle hypodensity mainly in the left occipital lobe, with a characteristic empty delta sign (Fig. 1). Computed tomography angiography demonstrated normal filling of the posterior cerebral arteries (PCA). Cranial magnetic resonance imaging (MRI) evaluations demonstrated bilateral ischemic changes involving the occipital and parietal lobes, more extensively on the left (Fig. 2). Magnetic resonance venography (MRV) demonstrated complete occlusion of the left transverse and sigmoid sinuses, as well as near occlusion of the posterior superior sagittal sinus (Fig. 3C).
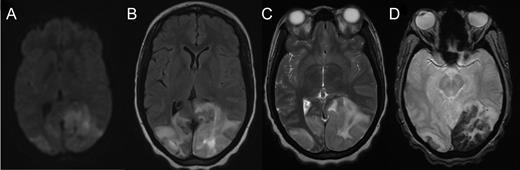
Axial MRI demonstrating cerebral tissue injuries from venous hypertension with the associated ischemia: (A) Diffusion-weighted imaging (DWI) MRI. (B) Fluid-attenuated inversion recovery (FLAIR) imaging. (C) T2-weighted MRI. (D) Gradient-echo (GRE) imaging.
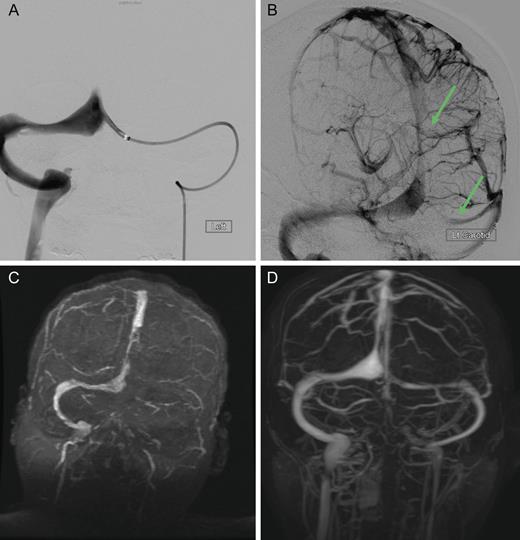
Dural sinus venogram (A) Prior to endovascular mechanical thrombectomy (B) Post mechanical thrombectomy. (C) Pre-intervention MRV demonstrating complete occlusion of the left transverse sinus and near occlusion of the posterior portion of the superior sagittal sinus. (D) Post-intervention MRV demonstrating persistent recanalization of the left transverse and sigmoid sinuses, along with much improved flow in the posterior superior sagittal sinus. Note the recanalization of the left transverse sinus and the much improved flow in the superior sagittal sinus.
The patient was treated emergently with endovascular mechanical thrombolysis with technical success (Fig. 3A and B), followed by systemic anticoagulation therapy. Post-intervention MRV demonstrated persistent recanalization of the left transverse and sigmoid sinuses, along with much improved flow in the posterior superior sagittal sinus (Fig. 3D).
She was subsequently diagnosed with mixed connective tissue disease. Clinically, she recovered the left visual field but continued to have a dense right homonymous hemianopsia at discharge. She returned to her home state and was unfortunately lost to further follow-up.
DISCUSSION
CVT comprises 0.5–1% of all strokes [2]. First described in 1825, CVT occurs much less frequently than arterial thrombosis but can present in an atypical fashion leading to misdiagnosis or delayed diagnosis [1]. Cortical blindness represents a rare presentation of CVT, and has not been well reported in the literature.
While CVT can present at all ages, it is more common in young adults and neonates. CVT primarily affects females (typically women of childbearing age), and recent reports indicate an increased frequency attributed to use of oral contraceptives [3]. Major causes of CVT constitute prothrombotic conditions such as thrombophilia, pregnancy and puerperium; the use of certain medications such as oral contraceptives or substance abuse; infections and head trauma [3]. In 20–35% of CVT cases, however, the etiology is unknown.
Patients with CVT commonly present with headache, papilloedema, motor or sensory deficits, seizures or conscious disturbance. Headache is the most common symptom, present in more than 90% of patients either in a gradual manner or as a sudden onset of severe headache (thunderclap headache). Only half (40–60%) of CVT patients develop subsequent cerebral lesions and neurologic deficits. Focal seizures have been noted to develop in 30–40% of CVT cases, which is much higher than in patients with arterial stroke [3].
Diagnosis of CVT is substantiated with neuroimaging. An abnormal sinus signal coupled with an absence of flow on MRV strengthens the diagnosis. If diagnosis is still uncertain at this stage, cerebral angiography may be attempted as it provides definitive information of the cerebral arterial and venous system. An empty delta sign is the most commonly observed sign of CVT on CT imaging (such as our patient), but this is only present in 20% of cases.
Main treatment options are anticoagulation, fibrinolytic therapy, thrombolysis (either directly or mechanically) and/or management of intracranial pressure [3]. Heparinization offered mortality rates typically <10%, which was often attributed to the underlying disease rather than CVT. If the patient experiences a raised intracranial pressure or worsened clinical state after anticoagulation, thrombolytic therapy is suggested. Presenting without light perception in either eye, our patient was treated emergently with mechanical thrombolysis followed by anticoagulation therapy (intravenous heparin bridging to coumadin), resulting in the successful recanalization of the left transverse sinus and a much improved flow in the superior sagittal sinus.
Visual loss is quite rare as the initial presentation of CVT and occurs more frequently in chronic cases with associated papilledema on funduscopy. Cortical blindness denotes a loss of vision due to damage on the geniculocalcarine tract, particularly the primary visual cortex (V1) [4]. This causes conscious vision deficit that may become permanent without timely intervention. It is often associated (40–90% of all cases) with a bilateral PCA occlusion [4]. Bilateral infarctions, edema, hemorrhage, thrombosis, trauma, meningioma, glioma and neurological disease involving the occipital cortex are some primary causes of cortical blindness in patients. In cortical blindness with vascular origins, loss of vision is often precipitous with some patients experiencing visual agnosia, hallucinations, denial or disorientation. Homonymous hemianopsia, both unilateral and bilateral in nature, have been described in patients with cortical blindness.
In our patient, the combined occlusion of the posterior third of the superior sagittal sinus and the left transverse sinus likely resulted in severe venous hypertension affecting the bilateral visual cortices with subsequent incomplete infarction. Incomplete bilateral infarction of the visual cortices can present with a remarkable variety of syndromes, with some cases having begun as complete blindness. Although patients with complete cortical blindness commonly volunteer no complaints and are unaware of the deficit [5], our patient acutely presented with the primary complaint of bilateral visual loss.
CVT presents in a remarkably wide spectrum of symptoms and remains a diagnostic and therapeutic challenge for clinicians. Although cortical blindness is most commonly caused by bilateral PCA occlusion, it can be a rare initial presentation of CVT.
Conflict of interest statement
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.



