-
PDF
- Split View
-
Views
-
Cite
Cite
Kazuma Noguchi, Hiromitsu Kishimoto, Koji Yamanegi, Kuniyasu Moridera, Kazuki Takaoka, Masahiro Urade, Unicystic ameloblastoma metastasizing to multiple cervical lymph nodes, Journal of Surgical Case Reports, Volume 2013, Issue 5, May 2013, rjt033, https://doi.org/10.1093/jscr/rjt033
Close - Share Icon Share
Abstract
Ameloblastoma is the most common odontogenic tumor, but the incidence of its metastasis is extremely low. We report a case of unicystic ameloblastoma metastasizing to the cervical lymph nodes. This patient pointed out a radiolucent cystic lesion with impacted wisdom tooth in the left mandibular region, and recieved enucleation of the cystic lesion and removal of the wisdom tooth. Histopathogical diagnosis was unicystic ameloblastoma. Three years later, this patient complained of a swelling in the left submandibular region. A CT scan showed a bilobed cystic mass measuring 30 mm in diameter compressing the submandibular gland, and we performed extirpation of the mass with the submandibular gland and associated lymph nodes. Histologically, the lesion was cystic and lymph follicles were seen in the cyst-like wall. The laminated epithelium of cyst wall was ameloblastomatous epithelium, and two lymph nodes associated with cystic lesion also included ameloblastomatous epithelium. This is the first report of metastasizing unicystic ameloblastoma.
INTRODUCTION
Ameloblastoma is the most common odontogenic tumor, and it is defined in the most current (2005) WHO classification of odontogenic tumors as a ‘slow-growing locally invasive epithelial odontogenic tumor of the jaws with a high rate of recurrence if not removed adequately, but with virtually no tendency to metastasize’ [1]. However, it has been reported that ameloblastoma has a substantial ability to develop metastasis in regional lymph nodes and distant organs [2]. Incidence of malignancy/metastasis ameloblastoma was earlier reported as 2%, but more realistically is far less [3].
Here, we report the first case of unicystic ameloblastoma metastasizing to the cervical lymph nodes, and discuss the route of metastasis in this benign tumor.
CASE REPORT
In 2009, a 26-year-old Japanese female patient was referred to our clinic, presenting with radiolucent cystic lesion in the left mandibular region. On oral examination, no swelling appeared to gingiva of the lower left second molar region. Neither paresthesia of the mental region nor palpable lymphadenopathy was observed. Panoramic radiography showed a radiolucent cystic lesion measuring 15 mm in diameter with impacted wisdom tooth (Fig. 1A). CT revealed a cystic lesion surrounding the crown of the wisdom tooth (Fig. 1B). Under clinical diagnosis of a dentigerous cyst, enucleation of the cystic lesion and removal of the wisdom tooth were performed (Fig. 1C). Histopathogical diagnosis was unicystic ameloblastoma (Fig. 2A and B). The postoperative course was uneventful.
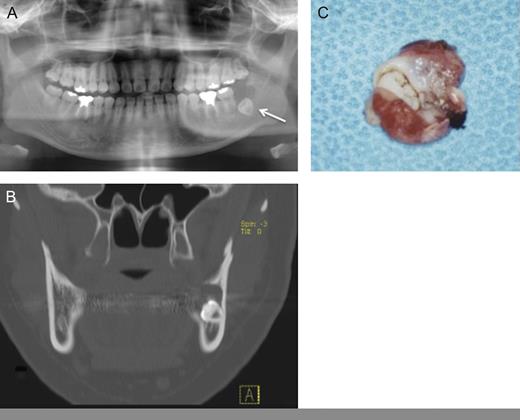
(A) Panoramic radiography: a cystic lesion with wisdom tooth was seen in the left mandibular region. (B) CT showed a cystic lesion with bone destruction. (C) Gross appearance of surgical specimen showed like dentigerous cyst.
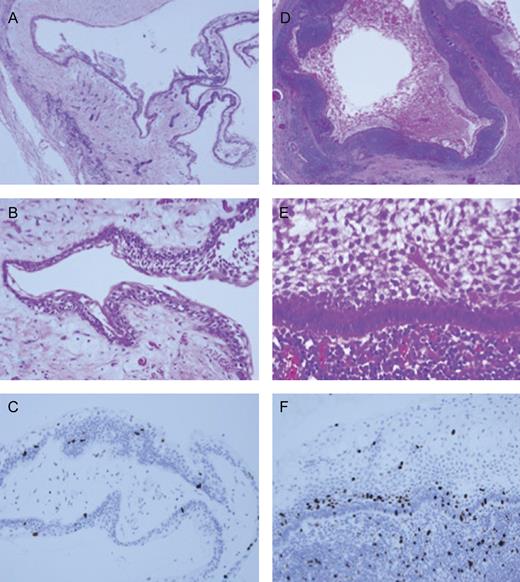
Histopathological findings. (A; ×40) and (B; ×200) showed pathological findings of primary unicystic ameloblastoma and (D; ×20) and (E; ×300) showed of metastatic lymph node. (C; ×200) and (F; ×300). Immunochemical staining of Ki-67 (MIB-1) revealed that the metastatic lesion was relatively high indexes compared with primary lesion.
In May 2012, this patient complained of a swelling at the left submandibular region of 2 months' duration (Fig. 3A). Physical examination showed a soft, mobile mass 30 mm in diameter in the submandibular area, and there was no clinical and radiographic sign of recurrence in the left mandibular lesion in the follow-up period (Fig. 3B). A CT scan with contrast medium showed a bilobed mass-like mucocele compressing the submandibular gland (Fig. 3C). MRI revealed that the contents of this mass were fluidic. A chest X-ray showed no abnormal finding. The patient underwent extirpation of a cystic mass with the submandibular gland and the associated lymph nodes (Fig. 3D). The submandibular mass showed a smooth surface covered with this capsule, and its contents was yellowish, transparent, mucinous liquid. The final histopathological diagnosis was consistent with ameloblastoma encapsulated in the mass and encapsulated with two metastatic lymph nodes. The patient had an uneventful postoperative course. In July 2012, the patient received CT scan of the mandible, neck and chest, and all regions were disease-free. The patient was placed on a strict follow-up schedule, with annual CT and chest X-ray. At the follow-up, the patient maintained disease-free.
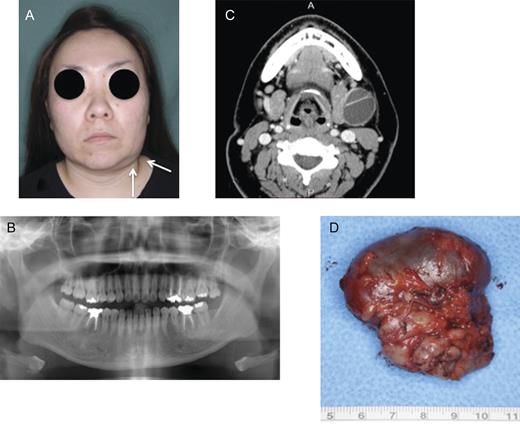
(A) Painless swelling of the left submandibular region occurred. (B) Panoramic radiography post-operation: no recurrence of ameloblastoma. (C) Computed tomography (C) showing a bilobed mass arising from the submandibular gland. (D) Macroscopic findings of extirpated submandibular gland including mass. The extirpated specimen had a smooth surface and a thin capsule. The contents of mass were yellowish, transparent, mucinous liquid.
Macro- and microscopically, the primary lesion was a typical cystic lesion including an unerupted wisdom tooth. Cyst wall consisted of ameloblastomatous epithelium which was laminated by palisading and nuclear polarized cells without any mitotic features (Fig. 2A and B). The lesion was diagnosed as a unicystic ameloblastoma, intraluminal type. The submandibular mass was cystic and lymph follicles were seen in the cyst-like wall (Fig. 2D). The laminated epithelium of cyst wall was ameloblastomatous epithelium similar to that of primary tumor, and presented no atypia and mitotic figures (Fig. 2E). The ameloblastomatous tumors also were found in the cervical lymph nodes. Cell proliferative analysis of submandibular lesion by Ki-67 immunohistochemical staining, however, indicated obviously high index compared with primary lesion, and the index ratio is <5% in primary lesion but >10% in palisading and nuclear polarized cells of metastatic lesion (Fig. 2C and F). Therefore, final diagnosis of tumor was metastasizing unicystic ameloblastoma.
DISCUSSION
A clear distinction between metastasizing ameloblastoma and ameloblastic carcinoma was made in the WHO classification [1], considering both the clinical behavior of metastasis and histopathological features. In this classification, metastasizing ameloblastoma is defined as an ameloblastoma that metastasizes in spite of a benign histologic appearance. It does not show any features that can be distinguished from ameloblastoma that does not metastasize and is reclassified as such only in retrospect when metastasis occurs. Ameloblastic carcinoma has combined histologic features of ameloblastoma and cytologic atypia with or without metastasis [4]. The lungs are the commonest site of metastasis, and cervical lymph nodes are the second most frequent site for metastatic ameloblastoma. Median survival after cervical metastasis of ameloblastoma is 6 years and 5-year survival rate 62% [5]. Unicystic ameloblastoma is an ameloblastoma variant, and 5–15% of all ameloblastomas are of the unicystic type [1]. Most ameloblastomas metastasize from solid/multicystic type ameloblastoma of mixed or pure plexiform pattern [6], and there are no reports of metastasis from unicystic type. The reason why this less aggressive lesion metastasizes to cervical lymph nodes has not been fully understood.
Generally, the route of metastasis could be via lymphatics, or hematogeous spread through blood circulation to regional lymph nodes or distant organs. On lung metastasis of ameloblastoma, implantation, as in aspiration, and growing on the surface of the lung, is also implicated [7]. Eisenberg [8] explains the occurrence of ameloblastoma in regional lymph nodes by the biologic process of heterotopy. According to her hypothesis, odontogenic epithelium becomes entrapped in lymphoid tissue during embryogenesis and may subsequently undergo benign neoplastic transformation. This result could be the development of an ameloblastoma within a cervical lymph node (i.e. a ‘second primary’), rather than metastasis or local spread to the lymph node from a primary ameloblastoma. Although histopathological features were almost identified as the primary tumor with metastatic lymph node, cell proliferative index by Ki-67 immunohistochemical staining revealed relatively high scores compared with that of primary lesion in our present case. This result suggested that metastatic lesion acquired a characteristic of biological aggressiveness, and it is difficult to agree with the hypothesis of heterotopy. We could not identify the metastatic pathway of this case, but suggested that main pathway of metastasis was via lymphatics.
The management of metastasizing ameloblastoma has been only surgical treatment, and cervical lymph nodes metastasis of ameloblastoma is to be treated by some type of a neck dissection [9].
This is the first case report of unicystic ameloblastoma metastasizing to the cervical lymph nodes. The present case suggested that the ameloblastoma possesses the potential for metastasis irrespective of histopathological classification.



