-
PDF
- Split View
-
Views
-
Cite
Cite
Kenza Oqbani, Ghassan El Omri, Amal Rami, Anass Idrissi, Synchronous colonic metastasis of sarcomatoid chromophobe renal cell carcinoma. Diagnostic challenges and value of CD10 and vimentin immunostains in diagnosis, Journal of Surgical Case Reports, Volume 2025, Issue 3, March 2025, rjaf145, https://doi.org/10.1093/jscr/rjaf145
Close - Share Icon Share
Abstract
In chromophobe renal cell carcinoma (CRCC), sarcomatoid and rhabdoid features denote a dismal prognosis, a short time to recurrence, and metastatic disease after nephrectomy. A 58-year-old male presented for management of a septic shock secondary to stercoral peritonitis. Four months before, he underwent a right nephrectomy for a lower polar renal tumor. The final diagnosis concluded to a pT4 CRCC with extensive sarcomatoid and rhabdoid changes. An abdominal CT-scan objectified a heterogeneous colon mass without renal recurrence. Urgently, the patient underwent right hemicolectomy and segment-VI hepatectomy. Histology suggested high-grade sarcoma with undifferentiated tumor proliferation and a lack of well-differentiated epithelial component. Immunohistochemistry (IHC) confirmed the renal origin of the colon metastasis, which consisted solely of the dedifferentiated component of CRCC. Tumors originating from the kidney should be considered in the differential diagnosis of undifferentiated colorectal cancers. An excellent anatomo-clinical correlation and a suitable IHC workup would reveal the appropriate diagnosis.
Introduction
Chromophobe renal cell carcinoma (CRCC) is a less common subtype of RCC, accounting for ~5%–10% of cases [1]. The incidence of sarcomatoid–rhabdoid dedifferentiation is estimated at 2%–11% in CRCC across series [2, 3]. These features are marked by their malignant biological behavior, intense proliferative activity, frequent p53 gene mutations, and the tendency to invade other organs [4].
Concomitant RCC metastasis is detected in over one-third of patients at diagnosis. The most common sites for metastases from RCC include the lung, lymph node, bone, liver, and brain. Colon metastasis of RCC is extremely uncommon [5]. In English literature, no case of early sarcomatoid CRCC colon metastasis arising following nephrectomy for a CRCC with extensive sarcomatoid changes has been reported in the literature. We present a case of peritonitis that revealed a colon metastasis from a dedifferentiated CRCC component. This metastasis developed during the first 6 months of primary CRCC diagnosis. Our case sheds light on the susceptibility of CRCC to progress to a high-grade malignancy with sarcomatoid and/or rhabdoid features.
Case presentation
A 58-year-old male presented to the emergency department of Sheikh Khalifa International University Hospital for management of stercoral peritonitis. His surgical history included right nephrectomy 4 months earlier for lower polar kidney tumor diagnosed as pT4 CRCC, ISUP grade-4 with extensive tumor necrosis and sarcomatoid-rhabdoid changes (65%), and extensive. The abdominal CT-scan objectified a heterogeneous colon tumor without renal recurrence (Fig. 1A). Moreover, a second examination of the first abdominal CT-scan realized before the nephrectomy confirmed the presence of a localized heterogenous right renal mass pushing the ascending colon without any infiltration (Fig. 1B). Urgently, the patient underwent a right hemicolectomy with segment-VI hepatectomy.
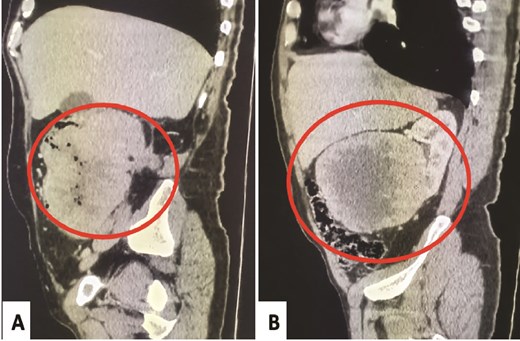
Abdominal CT-scan post-nephrectomy: Heterogeneous colonic tumor without renal recurrence (A). Initial abdominal CT-scan: Right renal mass pushing the ascending colon without any evidence of invasion (B).
Grossly, the colon specimen revealed a circumferential red-brownish polypoid and stenotic mass perforating the colonic serous and measuring 23 × 16 × 7 cm (Fig. 2A). It had significant hemorrhagic and necrotic changes (Fig. 2A). Hematoxylin and eosin-stained sections objectified an undifferentiated tumor proliferation suggesting a high-grade sarcoma (Fig. 2B). Surgical margins, liver VI segment, and lymph nodes were free of tumor.
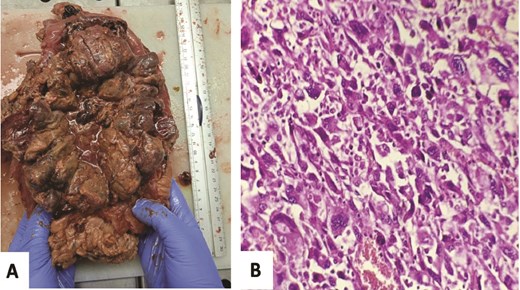
Gross examination: Red-brownish polypoid and stenotic mass with significant hemorrhagic and necrotic changes (A). Microscopic analysis: Undifferentiated proliferation showing pleomorphic large cell without any epithelial carcinoma component, especially no conventional CRCC pattern seen (HE, 400×) (B).
Immounohistochemistry (IHC) showed a diffuse and intense immunoreactivity for CD10 (Fig. 3A) and vimentin (Fig. 3B), confirmed the renal origin of the sarcomatoid component and ruled out other differential diagnoses by negative immunostains for keratin markers (AE1/AE3, EMA, and CK7), PAX8, Dog1, CD117, SMA, Desmin, myogenin, S100, and CD45 antibodies. The review of previous microscopic slides from nephrectomy showed CRCC with extensive rhabdoid and sarcomatoid features. These dedifferentiated components were morphologically similar to the colon tumor. The patient underwent palliative chemotherapy and remained disease free a few months later before he was lost to follow-up.
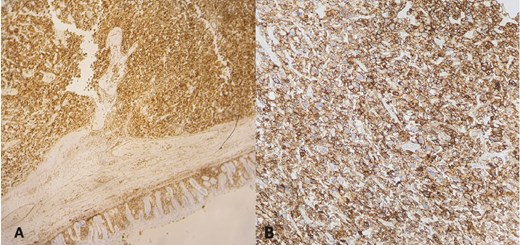
Immunohistochemical staining: diffuse and strong immunoreactivity of tumor cells for CD10 (A) and vimentin (B).
Discussion
In a study of 952 cases, the incidence of sarcomatoid differentiation was 9% in CRCC, 8% in clear cell renal carcinoma (CCRCC), and 3% in papillary renal carcinoma [6]. In general, high-risk pathological features include sarcomatoid and/or rhabdoid changes, pT stage, and tumor necrosis [5]. These factors modify the disease biology to a high-grade aggressive profile, leading to early recurrence with metastatic disease after curative nephrectomy and poor overall survival as illustrated in our case [7]. The majority of patients with sarcomatoid CRCC developed de novo metastatic disease [7]. When sarcomatoid features were present in the nephrectomy specimen, the median interval from surgery to metastatic recurrence was significantly shorter (31 months) [6]. Therefore, physicians should be aware of these data to plan a close follow-up [6]. Our patient developed colonic metastasis 4 months after nephrectomy.
In 35% of RCC cases, synchronous metastasis is present at the time of the primary RCC diagnosis, and ~30% may develop metachronous disease, leading to 10% of late diagnosis [8]. According to a literature analysis, Kataoka et al. revealed that the median time from primary tumor diagnosis to metastasis is 5 years, but it might range from months to years [9]. In our case, the metastasis to the colon was synchronous, as it appeared 4 months post-nephrectomy. It was not a locoregional recurrence as there was no tumor in the renal lodge. It is essential to distinguish rhabdoid/sarcomatoid features from other malignant tumors that exhibit similar morphology, such as gastro-intestinal soft tissue tumor (GIST), metastatic pleomorphic rhabdomyosarcoma, melanoma, lymphoma, and undifferentiated carcinoma. In our case, microscopic features were quite similar to high-grade sarcoma. However, the IHC study supported the diagnosis and underlined its importance for an appropriate diagnosis.
Classic CRCCs typically express diffuse and uniform CK7 and CD117 positive expression, which may be diagnostically useful for a young pathologist [10]. Sarcomatoid CRCCs exhibit decreased expression of these markers in dedifferentiated component areas; otherwise, they tend to lose CK7 but gain vimentin expression [10]. According to the literature, while CRCCs were negative for vimentin, most sarcomatoid cells (SCs) expressed it at positive rates ranging from 56% to 100% [11]. Furthermore, in 32 cases, SCs expressed strongly CD10 [12]. In our case, SCs expressed strong positivity for vimentin and CD10 antibodies. Chang et al. observed that PAX8 was positive in 69% of sarcomatoid RCC cases [13]. PAX8 was negative in our case. Alpha methylacyl CoA racemase (AMACR) was rarely expressed in SCs [12]. Among 42 cases of RCC with sarcomatoid differentiation, Yu et al. demonstrated the expression differences of CD10, vimentin, CK7, and CD117 between CCRCCs and CRCCs in carcinoma and sarcomatoid differentiation [13]. In this study, none of the carcinoma components of CRCC cases expressed CD10 (0/7) or vimentin (0/7). However, the sarcomatoid components were positive for CD10 and vimentin in 5/7 and 7/7 of cases, respectively [12].
In patients with RCC colonic metastases, the overall 5-year survival rate can be <10%, but surgical resection can increase it to 88%. Survival improvement can be demonstrated in multifocal metastasis [14].
Patients with lower GI symptoms or peritonitis, particularly those with a history of kidney mass, should be thoroughly screened to look for RCC metastasis, using physical examination and imaging modalities. In order to identify sarcomatoid CRCC from its mimickers, a comprehensive microscopic and immunohistochemical examination represents an optimal baseline strategy, along with prompt and appropriate multidisciplinary management. Genomic profiling can help diagnose and provide specific care for challenging patients. Finally, we propose curative resection for patients with solitary colon metastasis because it improves survival.
Author contributions
Kenza Oqbani (Conception and design, Collection and assembly of data, data analysis and interpretation, Manuscript writing), Ghassan El Omri and Anas Idrissi (Provision of study materials or patients), Amal Rami (Radiology images with annotations), and all authors (Final approval of manuscript).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding
None declared.
References
- computed tomography
- immunohistochemistry
- heterogeneity
- colonic neoplasms
- differential diagnosis
- hepatic resection
- kidney neoplasms
- neoplasm metastasis
- nephrectomy
- neprilysin
- peritonitis
- sarcoma
- vimentin
- abdomen
- colon
- diagnosis
- kidney
- neoplasms
- chromophobe carcinoma
- colectomy, right
- immunostain
- metastasis to the colon



