-
PDF
- Split View
-
Views
-
Cite
Cite
Mohammed F Shaheen, Fahad Aljehaiman, Abdulrahman Altheaby, Semi-simultaneous hand-assisted laparoscopic (HAL) bilateral nephrectomy and kidney transplantation from the same incision in ADPKD, first case report in Saudi Arabia, Journal of Surgical Case Reports, Volume 2024, Issue 5, May 2024, rjae274, https://doi.org/10.1093/jscr/rjae274
Close - Share Icon Share
Abstract
This case report discusses the management of a 46-year-old male patient with autosomal dominant polycystic kidney disease and a high body mass index, who underwent a semi-simultaneous procedure involving hand-assisted laparoscopic bilateral nephrectomy to alleviate severe abdominal symptoms and prepare for a kidney transplantation, all using the same incision. This is the first reported occurrence of such a procedure in Saudi Arabia. Post-operatively, the patient made a successful recovery with excellent kidney function and no complications.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a multisystemic disorder that primarily affects the renal system leading to bilateral cystic enlargement of the kidneys, early-onset hypertension and progressive renal deterioration resulting in end-stage renal disease (ESRD) in most affected patients [1]. A high incidence rate of ADPKD is found in Saudi Arabia with the presence of genetic variants that leads to earlier onset and progression to ESRD [2]. Although kidney transplantation is considered the definitive intervention at the time of renal failure, the need and optimal timing of native nephrectomy remain a topic of debate [3]. Few published papers discussed different approaches encompassing staged and simultaneous procedures [4]. Although simultaneous procedures offer an abbreviated approach to the patient, it is believed to harbor higher complications [5].
Herein, we described a semi-simultaneous approach involving hand-assisted laparoscopic bilateral nephrectomy (HALBN) and kidney transplantation (KT) from the same incision in a symptomatic and preemptive ADPKD patient. The adopted strategy marks the initial Saudi experience and is thought to offer a safe approach for early experiences in transplant centers.
Case report
A 46-year-old Saudi male with a history of ADPKD and ESRD was assessed for a living-related kidney transplant due to his significantly enlarged kidneys, causing discomfort and limited space to implant a new kidney (Fig. 1). He weighed 111.1 kg—reflecting a BMI of 38 kg/m2—with a serum creatinine of 783 μmol/L and an eGFR of 7 ml/min/1.73 m2.
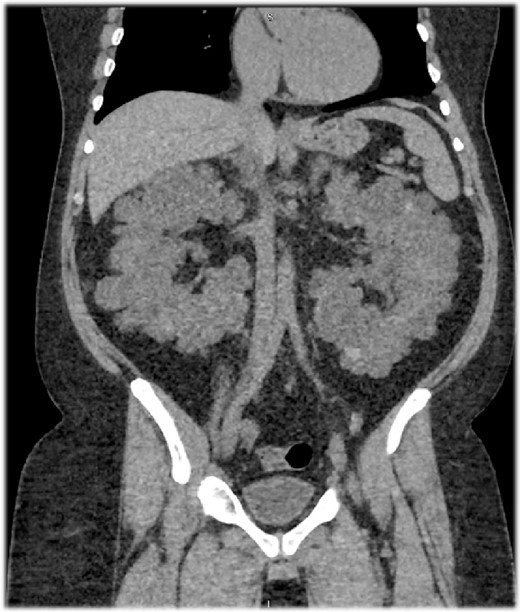
A sagittal section of a non-contrast computed tomography (CT) scan showing enlarged polycystic kidneys with the left and right kidneys measuring 16.5 × 10.1 × 11.3 cm3 with an estimated volume of 1.35 L and 16 × 9.4 × 13 cm3 with an estimated volume of 1.22 L, respectively. Both kidneys were filled with numerous cysts, replacing the normal renal parenchyma.
On October 2023, he underwent HALBN to manage symptoms and create space, followed by a living related KT the next morning. The patient was monitored in the intensive care unit in the interim. The extraction incision for the native kidneys was a modified Pfannenstiel incision, later used for the transplant surgery. To facilitate extraction without extending the incision, the cysts were crushed with a Kelly clamp (Fig. 2).
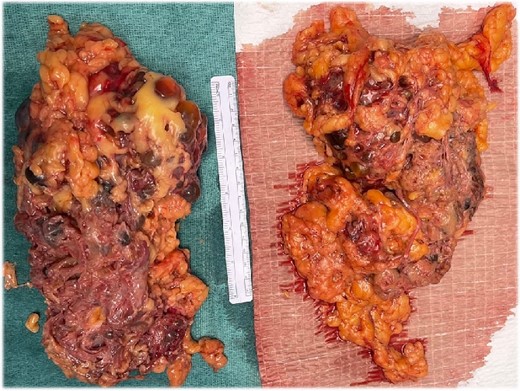
The gross appearance of the native kidneys after extraction. The Pathology report revealed presence of multiple cysts, most were empty, the rest were filled either with clear fluid or gelatinous material. No masses were identified.
The next morning, the KT incision was formed by connecting the prior extraction port and a laparoscopic port in the right lower quadrant (Fig. 3). The transplant resulted in immediate graft function with a warm ischemia time of 39 min and cold ischemia of 2 h and 44 min. By the fifth post-operative day, the patient was discharged with good urine output and normal serum creatinine levels, marking the procedures’ success.
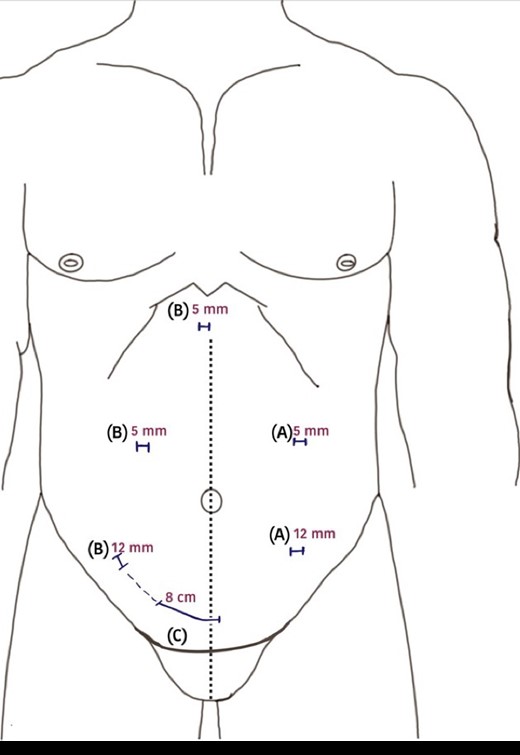
A diagram of the surgical incisions used during surgery. (A) 5-mm camera port and 12-mm working port position for left side nephrectomy; (B) 5-mm camera port, 12 mm working port and another 5-mm port used for liver retraction positions for right-sided nephrectomy; (C) the lateral end of Pfannenstiel incision extended to the right-sided 12-mm port incision on the day of kidney transplantation.
Discussion
Fortunately, the majority of patients with ADPKD and ESRD do not require nephrectomy [6–8]. However, those who do are often the more complex surgical cases with a higher propensity for complications, which can potentially compromise KT outcomes [9–12]. This advent of minimally invasive techniques has increased the demand for surgical expertise but has also resulted in fewer complications for patients [13, 14].
While simultaneous nephrectomy and transplantation procedure offers convenience and reduced operative time, it is not without risks. Potential complications, such as hypotension from blood loss, third-space fluid shifts, temporary adrenal insufficiency or disruption of the renin-angiotensin system, can complicate the post-operative recovery [15, 16]. Furthermore, a minimally invasive technique increases the demand for surgical expertise to minimize potential complications in such cases [10, 15]. Conversely, staged procedures may lead to an overall higher rate of complications, extended periods on dialysis and necessitate separate incisions for each surgery [4].
The minimally invasive semi-simultaneous approach advocated in this report presents a balanced solution. With careful surgical scheduling, it ensures a robust recovery and favorable cosmetic outcomes (Fig. 4). This strategy provides a buffer against hemodynamic instability or unexpected intraoperative complications that could arise from the nephrectomy while able to achieve outcomes comparable with simultaneous surgery. The drawbacks include the logistical challenge of arranging separate operative days and the requirement for two anesthesia events. This semi-simultaneous approach is ideal for centers new to this surgical option or when different teams handle nephrectomy and transplantation. The case report also reflects Saudi Arabia’s initial experience in such procedures.
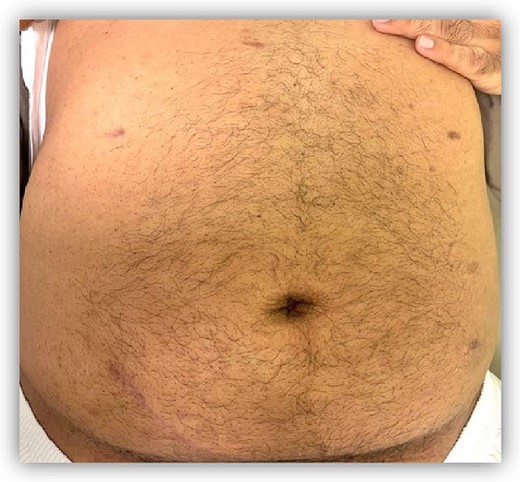
All skin incisions were closed in a subcuticular fashion yielding cosmetic scars 2 months post-operatively.
Conflict of interest statement
None declared.
Funding
None declared.



