-
PDF
- Split View
-
Views
-
Cite
Cite
Yeon Soo Kim, Life-threatening hemothorax due to rupture of pulmonary arteriovenous malformation during pregnancy, Journal of Surgical Case Reports, Volume 2024, Issue 3, March 2024, rjae139, https://doi.org/10.1093/jscr/rjae139
Close - Share Icon Share
Abstract
Pulmonary arteriovenous malformation (PAVM) can worsen in pregnant women due to pregnancy-related physiological changes. If a PAVM ruptures, it can become life-threatening. A 24-year-old female patient at 22 weeks of gestation presented to the hospital with chest pain and dyspnea. A simple chest radiograph revealed that the right lung was almost completely collapsed due to massive pleural effusion, and the heart was displaced to the opposite side. Closed thoracostomy was performed and 2 l of blood was drained. Chest CT revealed the presence of a PAVM in the right upper lung. Emergency surgery was performed to resect the PAVM through thoracoscopic pulmonary wedge resection. The patient experienced disseminated intravascular coagulation and acute renal insufficiency after the surgery, but eventually recovered and was discharged without any complications on the sixth postoperative day.
Introduction
Pulmonary arteriovenous malformation (PAVM) is a rare condition, where the pulmonary artery and pulmonary vein are directly connected, resulting in a right-to-left shunt. Physiological changes during pregnancy can exacerbate PAVM. Increased blood volume and cardiac output can increase pulmonary flow [1, 2]. The rise in progesterone levels can also increase blood vessel dilatability, leading to the growth of PAVM [1]. These changes can cause thin blood vessels to dilate and possibly rupture. Here, we present a case of a rupture of a PAVM during pregnancy with a life-threatening hemothorax. Rapid identification and treatment of this condition can be life-saving.
Case reports
A 24-year-old female in the 21st week of pregnancy was transferred to the emergency department. Twelve hours before admission, the patient developed a tearing pain from the back to the right chest and dyspnea. There was no relevant medical history. Vital signs were systolic blood pressure of 83 mmHg, diastolic blood pressure of 58 mmHg, and pulse rate of 142/min. Initial hemoglobin level was 8.9 g/dl with a platelet count of 99 000/μl. Chest radiographs revealed a massive right pleural effusion with a mediastinal left shift (Fig. 1a). A closed thoracostomy was performed, and 2 l of blood was drained from the pleural cavity. Contrast-enhanced chest computed tomographic scan revealed a potentially ruptured PAVM in the anterior segment of the right upper lung (Fig. 1b). The patient was transfused with five packs of red blood cells and two packs of frozen fresh plasma. Emergency video-assisted thoracoscopic surgery (VATS) was performed. A 5-cm-sized working widow was created in the fifth intercostal space mid-axillary line. Two additional 10.5-mm ports were inserted in the sixth and seventh intercostal spaces in the anterior and posterior axillary lines. A half-liter of blood clots were removed. The PAVM, active bleeding on soft palpation, was visible on the right upper lung surface and was resected with two endostaples (Fig. 2). The patient developed disseminated intravascular coagulation and acute kidney injury postoperatively and was managed in the intensive care unit (ICU) for 3 days. She was recovered and was discharged 6 days after the operation without residual complications. The fetus had no particular problems related to the operation. However, it was miscarried after hydrocephalus and pericardial effusion were discovered. A follow-up computed tomographic scan 8 months after the operation revealed no residual PAVM (Fig. 3).
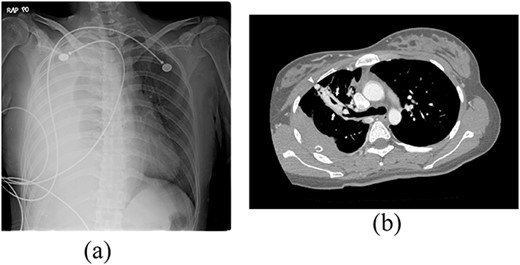
Radiograph of a 24-year-old woman with chest pain and dyspnea. (a) Chest radiograph showing right massive effusion causing left shifting of the mediastinum. (b) Enhanced axial CT showing the direct connection of a branch of the anterior segmental pulmonary artery (down arrow) with a vein (up arrow) and dilated vessel on the surface of the anterior segment of the right upper lung (arrowhead).
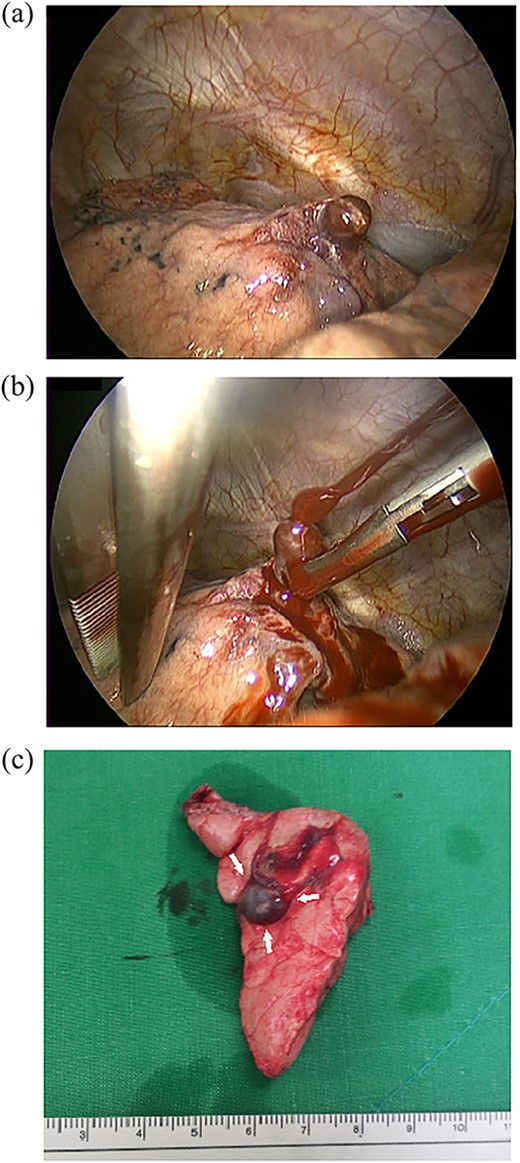
Operative findings. (a) The right upper lobe had a PAVM protruding from the anterior segment of the right upper lung. (b) Active bleeding from the PAVM. The PAVM is very friable and easy to rupture even with soft palpation. (c) Resected lung specimen. The PAVM is observed as a dilated blood vessel (arrow).
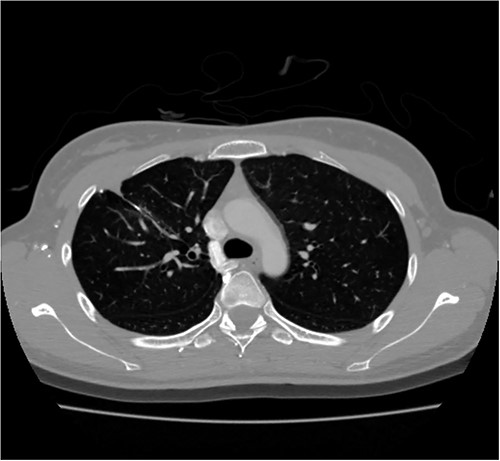
Contrast-enhanced chest CT after 8 months. Chest radiograph reveals no residual PAVM.
Discussion
Pulmonary arteriovenous malformations (PAVMs) are abnormal vascular structures that directly connect a pulmonary artery and a pulmonary vein without a normal capillary bed. It causes a right-to-left shunt that can lead to a decrease in oxygen saturation and an increase in paradoxical embolism by bypassing the natural filtration of the lungs. PAVMs are rare in the general population with a reported prevalence as low as 2–3 per 100 000 people with a male to female ratio of 1:1.5–1.8 [3]. PAVMs are most commonly found in individuals with the inherited vascular disorder hereditary hemorrhagic telangiectasia (HHT) [4, 5]. Common clinical symptoms associated with PAVMs include epistaxis, dyspnea, telangiectasia, cyanosis, and clubbing in the presence of a significant right-to-left shunt [6]. Paradoxical embolism may occur leading to transient ischemic attack, stroke, or brain abscess. Less commonly, PAVM may rupture spontaneously, resulting in a life-threatening pulmonary hemorrhage. This could potentially result in a massive hemoptysis or hemothorax. PAVMs are associated with significant morbidity and mortality [4].
Although there is no evidence on the growth rate of PAVMs, there appears to be an association between pregnancy and the spontaneous rupture of PAVMs. Pulmonary hemorrhage was reported in 8% (11 of 143 patients) of PAVMs associated with HHT. Eight of the 28 cases (29%) that occurred during pregnancy reported in the literature exhibited hemothorax associated with PAVM [4]. Physiological changes during pregnancy can worsen PAVMs and increase the risk of hemorrhage. Healthy pregnant female blood levels are almost 50% above non-pregnant levels. This represents an increase of ~1500 ml [2]. Both heart rate and stroke volume were increased in the pregnant woman. The cardiac output increased ~4 to 6 l/min [2]. The increase in blood volume and cardiac output during pregnancy results in increased pulmonary blood flow. The augmented pulmonary blood flow causes the dilation of PAVMs. The increased progesterone during pregnancy leads to smooth muscle relaxation in the arteries and veins, and a subsequent decrease in resistance across the PAVM, resulting in the dilatation of PAVMs. These physiological changes lead to an increased risk of hemorrhage [1].
Percutaneous transcatheter embolization (TCE) is the recommended first-line treatment of PAVMs because it reduces the risk of paradoxical embolisms and other complications. The advantages of the procedure are that it is less invasive and easy to repeat. Recanalization and collateralization can range from 5% to 19% [3]. The treatment of PAVMs is indicated when the feeding vessel(s) of the PAVM is (are) ≥3 mm in diameter. The treatment of smaller PAVMS (with a feeding vessel of 1.5–2 mm) is also justified when technically feasible and right-to-left shunting is present [7].
Surgical treatment for PAVMs before the method of blocking blood vessels using a catheter is currently rare. If catheter-based treatment is not appropriate for complex or multiple PAVMs, surgical lung resection of lobes or segments can be performed, and even in such cases, a conservative approach is necessary. Surgery may also be used in emergency situations, such as hemothorax [7].
In summary, this case involved a life-threatening hemorrhage that filled most of the right thoracic cavity after rupture of a PAVM. Emergency video-assisted thoracic surgery was performed to achieve hemostasis and excise the PAVM.
Conflict of interest statement
None declared.
Funding
None declared.



