-
PDF
- Split View
-
Views
-
Cite
Cite
Alex Mremi, Janeth Mpelumbe, Furaha Enos Kasyupa, Elizabeth Patrick, Orgeness Jasper Mbwambo, Bartholomeo Nicholaus Ngowi, A case report of two synchronous primary urologic malignancies in one patient, Journal of Surgical Case Reports, Volume 2024, Issue 3, March 2024, rjae130, https://doi.org/10.1093/jscr/rjae130
Close - Share Icon Share
Abstract
It is quite unusual to have numerous primary malignant tumors at the same time in the same patient. These cancers are classified as metachronous or synchronous. The occurrence of synchronous urologic tumors poses diagnostic and treatment challenges and has always been a subject of controversy in the clinical decision-making process. Unfortunately, no clear standardized management protocols for these patients exist. Therefore, diagnosis and treatment may be difficult, especially with few resources. We present a 75-year-old man with simultaneous prostate and kidney cancers successfully treated at our center. This is one of the rare cases in the English literature with two primary urologic cancers.
Introduction
Synchronous cancer is when multiple primary malignant tumors simultaneously occur in one patient [1]. Synchronous tumors must be present in different organ locations and have different histopathological characteristics [2, 3]. Each tumor must be primary, which eliminates the possibility of being metastatic. The occurrence of synchronous urologic cancers at the same time is unusual [4]. It may pose challenges in diagnosis and treatment, particularly in a resource-constrained setting [5]. Herein, we present a case of a 75-year-old male with prostate cancer and urothelial carcinoma of the kidney. A brief review of the literature is also provided.
Case report
A 75-year-old man presented with a chief complaint of lower urinary tract symptoms (LUTS) for 2 months. This entailed an increased urinary frequency, urgency, weak stream, and straining. The patient also reported back pain and painless gross hematuria that was initially intermittent. He also reported weight loss, coughing, easy fatigue, and right flank pain. He was hypertensive on regular medication. He was a peasant, and he has been smoking and drinking alcohol for many years.
On physical examination, the patient was afebrile, not pale, not jaundiced, and not cyanosed. Per abdomen, there were normal abdominal contours that moved with respiration; no organomegally was appreciated. On digital rectal examination (DRE), the patient had a normal anal verge with a normal anal sphincter tone and a hard, grade 3 nodulated prostate. The median sulcus was not obliterated. His vital signs were within normal limits. His laboratory test results revealed an elevated prostatic specific antigen (PSA) of 58.3 ng/mL (0.01–4.00) and a low hemoglobin of 9.4 g/dL (12–15). A cystoscopy results were normal. A clinical impression of prostate cancer was entertained. The patient underwent transurethral resection of the prostate (TURP) to treat symptoms of an enlarged prostate as well as histopathology. The biopsy results confirmed the diagnosis of invasive prostatic adenoma carcinoma; Gleason score 3 + 4 = 7 (Fig. 1).
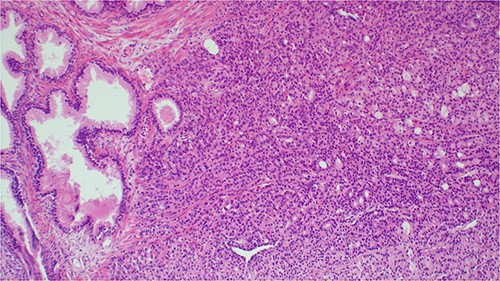
Histopathology image showing invasive prostatic adenocarcinoma, hematoxylin and eosin staining (H&E) staining 100× original magnification.
The chest and abdomino-pelvis CT scan demonstrated a well-defined tumor mass at the supero-posterior right kidney pole that measures 3.6 × 4.1 × 4.9 cm with peri-hilar lymphadenopathy (Fig. 2). Other visceral organs appeared normal. There was no evidence of metastasis. A diagnosis of renal cell carcinoma was suggested. The patient was counseled for a nephrectomy and bilateral total orchiectomy (BTO), which were successfully performed. He was kept on bicalutamide (casodex), antibiotics, and other supportive care.
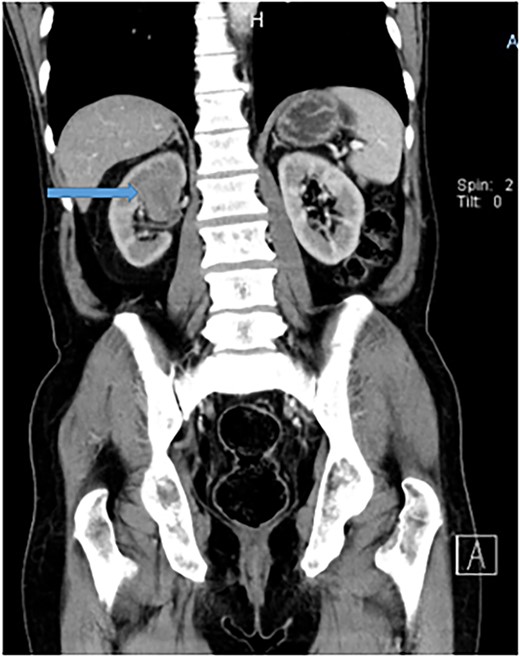
Abdomino-pelvis CT scan demonstrating a well-defined tumor mass at the supero-posterior right kidney pole that measures 3.6 × 4.1 × 4.9 cm.
Nephrectomy surgical procedure
The patient received prophylactic antibiotics prior to surgery. Under general anesthesia, the patient was placed in a supine position, cleaned, and draped aseptically. A urethral catheter was placed, a right transverse abdominal incision was made, and the abdomen was opened in layers. Intra-operatively, a right upper pole renal tumor extending up to the renal pelvis with necrotic peri-hilar lymphadenopathy was seen. The left kidney appeared normal in size and texture. Right-open nephroureterectomy and removal of the bladder cuff with peri-hilar lymph node clearance were done. The remaining lower ureter was disconnected using a separate incision in the lower abdomen. The abdomen was closed in layers with absorbable stitches, and a drain was left in situ. Histopathology of the rephrectomy specimen highlighted an invasive, high-grade tumor made up of purely atypical urothelial cells with high-grade nuclear features. Lympho-vascular space and capsular invasion were associated (Fig. 3). The tumor cells expressed immunopositivity with CK20 (Fig. 4), but were negative for PSA and vimentin. The diagnosis of invasive urothelial carcinoma, pT3N1M0, was established.
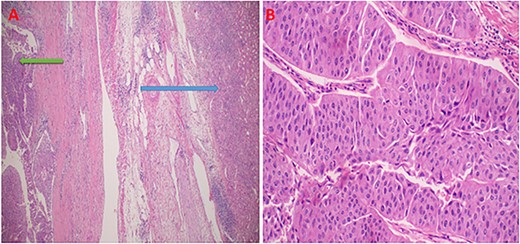
Photomicroscopy showing high grade renal urothelial carcinoma, and normal renal parenchyama; H&E staining 2 x original magnification (A); intermediate magnification of urothelial carcinoma displaying neoplastic cells in with high nuclear features in nests or papillary structures, H&E staining 10× original magnification (B).
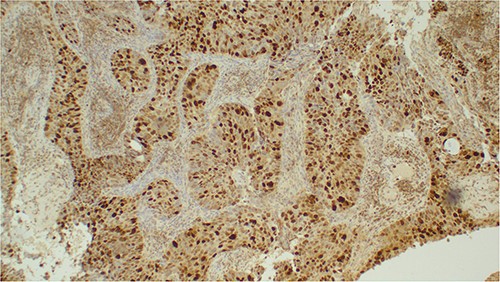
Photomicroscopic images of the urothelial carcinoma of the kidney demonstrating strong immunopositivity with CK20 immunohistochemistry; IHC 100× original magnification.
The patient was kept on adjuvant chemotherapy for renal urothelial carcinoma. This included docetaxel, gemcitabine, and carboplatin, and then he was discharged. During follow-up, the control PSA test was 1.710 ng/mL, suggesting a good biochemical response. To date, 12 months post-oncological treatment, the patient remains symptoms-free and in stable condition. The team of treating oncologists is considering radiotherapy for better disease control.
Discussion
The occurrence of synchronous cancers of the prostate gland and kidney is extremely unusual. Only a few cases have been published in English literature [4, 6]. However, their number appears to be rising, possibly due to advancements in screening, diagnostics, and oncological therapy [6–8]. Although the pathogenesis is unclear, various hypotheses have been postulated to try to explain the occurrence of this rare disease phenomenon. Simultaneous germ line nonsense mutations and environmental exposure to carcinogenic substances such as tobacco, asbestos, and ultraviolet light have been implicated as predisposing risk factors [6]. Other factors include familial cancer, advancing age, unhealthy lifestyles such as smoking, and alcohol use, most of which were seen in our patient.
Unlike prostate cancer, which is common, primary renal urothelial carcinoma is a rare tumor that mostly arises from the renal pelvis [6, 7]. It is critical to rule out metastasis before considering this diagnosis. A re-biopsy may be necessary whenever a suspicious new lesion is found in a cancer patient, either during treatment or surveillance after the treatment. As was the case with our patient, most patients are male, with a mean age of 70 years. Similarly, hematuria as a result of fragmentation within the renal pelvis is a common presentation. Contrary to the index case, most renal urothelial carcinomas typically present with pre-existing urinary bladder cancer [8]. Synchronous urologic malignancies often pose diagnostic and treatment challenges [9, 10]. Multi-disciplinary tumor board discussion by specialized physicians is essential to maximize treatment outcomes. The treatment approach should consider the type, stage, and grade of each tumor, as well as the functional status of the patient. The tumor posing the greatest threat to the patient’s survival or quality of life should receive priority care [11]. In the index case, BTO and open nephroureterectomy, which is the gold standard for the management of high-grade invasive upper tract urothelial carcinoma, were undertaken because they were appropriate and feasible, followed by chemoradiotherapy and endocrine therapy [12–14].
Conclusion
Synchronous urologic malignancies in a patient at the same time pose challenges to clinicians. Due to a lack of well-established standard treatment protocols, synchronous tumors have often been a subject of controversy in the clinical decision-making process. Therefore, further studies are warranted so as to improve management strategies.
Acknowledgements
The authors would like to thank the patient for allowing us to use his medical information for academic purpose. We equally acknowledge the support from the staffs in departments of Urology, Pathology, Radiology, and Oncology at the Kilimanjaro Christian Medical Centre, as well as the hospital management for their invaluable inputs in acquisition of information and other facilities that enabled the write up of this case study.
Conflict of interest statement
All authors have declared that no competing interests exist.
Funding
The work did not receive any fund from any source.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethical approval was waived by the authors’ institution.
References
https://www.orci.or.tz/wp-content/uploads/2020/02/National-Cancer-Treatment-Guidelines.pdf



