-
PDF
- Split View
-
Views
-
Cite
Cite
Cadric Gunaratnam, Olga Muzicenco, Premala Sivagurunathan, Michael Hogden Franzco, VKH-like syndrome in the setting of Dabrafenib and Trametinib therapy for BRAF mutant metastatic melanoma: a case report, Journal of Surgical Case Reports, Volume 2024, Issue 3, March 2024, rjae104, https://doi.org/10.1093/jscr/rjae104
Close - Share Icon Share
Abstract
Dabrafenib and trametinib, approved for the treatment of BRAF-mutant metastatic melanoma, are associated with a spectrum ophthalmic toxicity including pan-uveitis and serous retinopathy. Vogt–Koyanagi–Harada (VKH) is a systemic inflammatory disorder characterized by bilateral granulomatous pan-uveitis, exudative retinal detachments, and often associated with extraocular manifestations such as tinnitus, vitiligo, headaches, or encephalopathy. We present a 49-year-old woman with stage IV metastatic cutaneous melanoma developed bilateral acute pan-uveitis with multifocal serous retinal detachments, 4 months after starting combined dabrafenib and trametinib therapy. Clinical assessment, together with fluorescein angiography, optical coherence tomography, and serology led to the diagnosis of a (VKH)-like uveitis. Prompt systemic corticosteroids and modification of the dosing schedule of the suspected offending agents resulted in the resolution of intraocular inflammation and serous retinal detachments. This case underscores the importance of the prompt recognition of the association between VKH-like uveitis and BRAF/MEK inhibitors, enabling early intervention without compromising metastatic melanoma treatment.
Introduction
The survival rate of patients with advanced cutaneous melanoma has dramatically improved since the introduction of immune checkpoint and targeted therapy drugs [1]. Approximately 50% of melanomas contain an activating mutation in the BRAF oncogene, consequently triggering the MAPK pathway and resulting in uncontrolled cell proliferation and immune escape [2, 3]. Often used in combination, BRAF and MEK inhibitors have been shown to modulate the activity of the MAPK pathway, inhibiting cell proliferation and improving the survival rate [2, 3]. Dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) are a combination of targeted therapies approved for the treatment of BRAF-mutant advanced melanoma or metastatic melanoma. Vogt–Koyanagi–Harada (VKH) syndrome is a rare granulomatous autoimmune disorder primarily affecting the eyes, skin, and other melanocyte-rich tissues [4]. The clinical manifestations of VKH include uveitis, neurological symptoms such as headache and hearing loss, and cutaneous manifestations of vitiligo and poliosis [4]. With the increased use of combined BRAF and MEK inhibitors in the management of melanoma, multiple studies have reported various ocular adverse effects, including dry eyes, ocular and orbital inflammation, neuro-ophthalmic disorders, and serous retinopathy [1]. Treatment-related VKH-like syndrome is rare presentation but considered a severe ocular adverse event associated with combined therapy [4, 5].
Case presentation
A 49-year-old Caucasian female presented to our eye casualty with a 3-day history of bilateral blurred vision and an associated retro-orbital ache. Her medical history was significant for dyslipidemia, hypothyroidism and BRAF mutant stage IV metastatic melanoma commenced on dabrafenib and trametinib 4 months prior. Her restaging positron emission tomography (PET) scan performed 6 weeks prior to presentation illustrated no evidence of disease activity. She had no previous ocular history. There was no family history of ocular disease.
At the time of presentation, the best-corrected visual acuity (BCVA) was 6/9 + 3 in the right eye and 6/5–3 in the left eye. Intraocular pressures were within normal in both eyes. Optic nerve functions were normal. The anterior segment illustrated narrow occludable angles right more than left but otherwise was unremarkable. Dilated fundus examination of the right eye illustrated a hyperemic disc with mild nasal optic nerve head swelling (ONHS). The macula had evidence of an epiretinal membrane and few small choroidal folds. There was no evidence of retinitis or vitiritis. Fundus examination of the left eye illustrated a pink disc with subtle superonasal blurred margin but was otherwise unremarkable. Optical coherence tomography (OCT) illustrated mild subretinal fluid nasal to the optic nerve in the right eye and was otherwise unremarkable in the left eye. An urgent computed tomography brain and venogram done was unremarkable. In consultation with the patient’s oncologist, dabrafenib and trametinib were continued at this stage.
One week after initial review, the patient presented with eye casualty with deteriorated vision of 6/9 in the right eye and count fingers in the left eye. Intraocular pressure was within normal limits in both eyes. Anterior segment examination showed mild anterior uveitis with mild conjunctival injection, 1+ cells in the right eye, 2+ cells in the left eye, and 0.5+ cells in the anterior vitreous of both eyes. No keratic precipitants or posterior synechiae were present in either eye. Fundoscopy revealed bilateral hyperemic discs and 360° choroidal detachments with serous retinal detachment involving the maculae (Fig. 1). There was no evidence of vitritis or retinitis. OCT illustrated detachments of the neurosensory retina and bacillary layers (Fig. 2). Fundus fluorescein angiogram (FFA) demonstrated bilateral disk hyperfluorecence, macular hyperfluorescent pinpoints, and no evidence of retinal vasculitis or ischemia (Fig. 3).
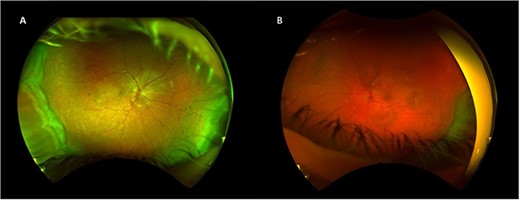
Right (A) and left (B) fundus examination illustrating hyperemic discs and circumferential choroidal detachments 1 week post initial presentation.
Here, we highlight a case of retinal toxicity secondary to combined BRAF and MEK inhibitors resulting in moderate bilateral pan uveitis with multiple serous retinal detachments.
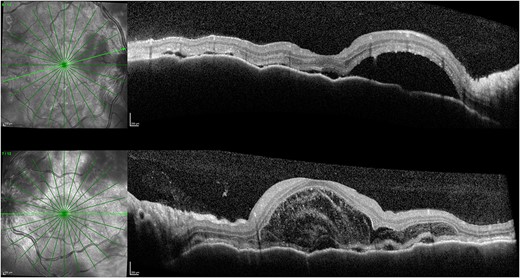
OCT 1 week after initial presentation illustrating neurosensory retinal and bacillary layer detachments, retinal pigment epithelium folds, and darkened choroid pathognomonic for VKH.
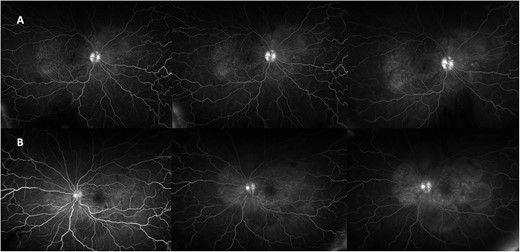
FFA demonstrating right (A) and left (B) disc hyperfluorecence and macular hyperfluorescent pinpoints, and pooling in areas corresponding to serous detachments.
Given the clinical features, the patient was diagnosed with VKH-like syndrome, presumably secondary to MEK and BRAF inhibitor therapy. In consultation with her oncologist, dabrafenib and trametinib were withheld. She was admitted and promptly started on pulsed methylprednisolone 1 g daily for 3 days. Autoimmune and infective screens were all negative. Magnetic resonance imaging of the brain with contrast had no evidence of cerebral vasculitis. The patient responded well to methylprednisolone with BCVA of 6/9–1 in the right eye and 6/30 in the left after 3 days. Subsequently, she was started on a slow tapering dose of oral prednisolone of 5 mg weekly with a starting dose of 65 mg daily. In conjunction with the oncologist, dabrafenib was reintroduced at 150 mg twice daily after being withheld for 2 weeks. Trametinib was reintroduced at a lower dose of 0.5 mg daily and was slowly up titrated to 1 mg after being withheld for 4 weeks. Her BCVA and OCT were monitored monthly, illustrating a progressive decline in ONHS and subretinal macular fluid. Four months later, her BCVA recovered to 6/6 in both eyes with no evidence of active uveitis and completely resolved subretinal fluid while on a maintenance dose of 10 mg prednisolone daily (Fig. 4).
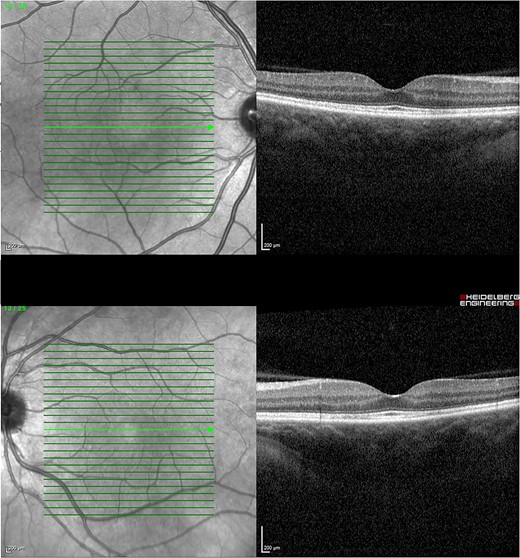
OCT 4 months post treatment showed complete resolution of fluid and reinstitution of normal retinal architecture.
Discussion
The utilization of immunotherapy and targeted drug treatment has emerged as the new standard of care for patients with unresectable or metastatic malignant melanoma, leading to improved overall survival rates [2]. This case highlights the complex interplay between oncological treatment and the potentially vision-threatening adverse events, particularly in the context of VKH disease. Within the literature, studies report systemic adverse events such as fatigue, nausea, arthralgia, reduced ejection fraction, and pyrexia [3, 6]. However, the prevalence and severity of ocular adverse effects related to combined dabrafenib and trametinib therapy continue to be poorly documented and are largely limited to clinical trials [4–6].
A recent retrospective review conducted by Mettler et al. looking a World health Organization global safety database aimed to characterize the ocular safety profile of BRAF, MEK, and combined inhibitors. BRAF inhibitor monotherapy was significantly associated with iris and ciliary abnormalities, anterior uveitis, and pan-uveitis [5]. MEK inhibitors monotherapy had significant association with retinal and choroid abnormalities, and pan-uveitis [5]. Mettler et al suggest increased risk of toxicity in combined targeted therapy, the most frequently documented was serous neuroretinal detachment and all types of uveitis [5]. In addition, the study illustrated increased reporting of VKH disease in the BRAF and combined therapy groups [5].
VKH disease is a rare disorder primarily characterized by bilateral panuveitis with serous retinal detachments secondary to autoimmunity to melanin-rich tissues [4]. In our case, though the patient lacked the typical granulomatous anterior reaction, the evolution of symptoms over the span of weeks and the clinical features of bilateral panuveitis, choroidal detachments, serous retinal detachments, and ONHS align with the classic features of VKH. The exact etiology of VKH-like syndrome in relation to dabrafenib and trametinib treatment is unclear, but some studies suggest the combined treatment leads to the formation of reactive oxygen species resulting in the breakdown of the blood retinal barrier [3]. Consequently, there is loss of the immune privileged ocular site, and potentially leading to autoimmune responses such as uveitis [3].
Tarim and Kilic [7], Lim et al. [6], and Brambati et al. [3], all describe varying presentations of VKH-like disease secondary to combined BRAF & MEK inhibitors in the treatment of malignant melanoma. All patients developed varying degrees of bilateral acute panuveitis and multifocal serous retinal detachments within 4 months combined therapy. These cases showed favorable visual prognosis with prompt cessation of chemotherapeutics and commencement of both topical and systemic corticosteroid treatment. This trend has also been observed in the case that we present within this report, with full resolution of symptoms within 6 months of treatment.
Conclusion
Both ophthalmologists and oncologists should remain vigilant for ocular manifestations in patients undergoing multiple targeted therapies, particularly when these therapies involve manipulation of immune signaling pathways. Swift recognition, discontinuation of the triggering agents, and timely initiation of appropriate immunosuppressive therapy were instrumental in achieving a favorable visual outcome for the patient.
Conflict of interest statement
None declared.
Funding
None declared.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Data availability
All data generated or analysed during this study are included in this published article.



