-
PDF
- Split View
-
Views
-
Cite
Cite
Santiago A Endara, Gerardo A Davalos, M Patricia Ponton, Christian I Gordon, Gabriel A Molina, Incidental finding of an ectopic b2 thymoma attached to the pericardium, Journal of Surgical Case Reports, Volume 2024, Issue 3, March 2024, rjae107, https://doi.org/10.1093/jscr/rjae107
Close - Share Icon Share
Abstract
Ectopic thymoma is a rare tumor that arises from the abnormal migration of thymus tissue. They are extremely rare and have a broad spectrum of clinical symptoms. Therefore, preoperative diagnosis is complex and can be easily misdiagnosed. Complete resection is the treatment of choice to avoid recurrence, radiotherapy and enhanced survival. Regretfully, many patients arrive at a late stage, limiting our therapeutic options; therefore, pre-operative diagnosis is vital. We present the case of an otherwise healthy 32-year-old woman; after a chest X-ray was done for a routine medical evaluation, a mass was discovered in her mediastinum. After surgery, a B2 thymoma attached to the pericardium was discovered and successfully treated.
Introduction
Thymomas are thymic epithelial neoplasms rarely found outside the anterior mediastinum [1]. Ectopic thymomas are not only extremely rare, but their symptoms are so atypical that clinical diagnosis is complex, and misdiagnosis occurs [1, 2]. Surgical resection is the best treatment to avoid recurrence and enhance survival [2, 3].
We present the case of an otherwise healthy young woman; after a chest X-ray was done for a routine medical evaluation, a mass was discovered in her mediastinum. After extensive medical evaluation and surgery, a B2 thymoma attached to the pericardium was found.
Case report
The patient is an otherwise healthy 32-year-old woman. In her youth, for about 3 years, she used to smoke 4–5 cigarettes a day but quit. During a routine medical examination, a standard chest X-ray was needed to obtain an international visa. It unveiled a 30 × 30 mm rounded opacity in the mediastinum; it was surrounded by normal lung parenchyma and was not associated with adenopathies, atelectasis or pleural effusion. Due to this, a contrast-enhanced chest computed tomography (CT) revealed a 38 × 29 × 28 mm heterogeneous solid mass. It was attached to the pericardium and surrounded by the trunk of the pulmonary artery; no other masses or lymph nodes were discovered (Fig. 1).
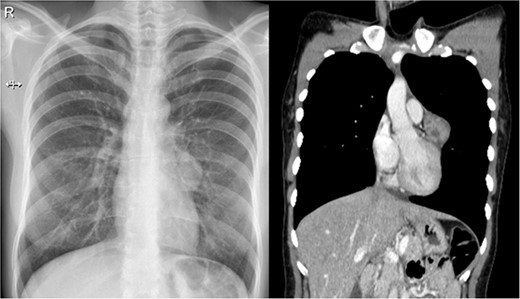
(a) Chest X-ray: a small rounded mass attached to the heart. (b) Chest CT shows a heterogeneous solid mass with well-defined borders close to the pericardium and near the trunk of the pulmonary artery.
A cardiothoracic assessment was needed, and the patient was transferred to our hospital. Physical examination was unremarkable; there was no palpable lymphadenopathy of the cervical, anterior mediastinal, supraclavicular, axillary or inguinal lymph nodes. Bilateral breath sounds were normal, and there was no history of fatigue, generalized pruritus, night sweats, fever, weight loss, cough, dyspnea or chest pain. Laboratory results were normal and nothing out of the ordinary; tumor markers, such as BHCG, AFP, CEA, CA19–9 and thyroglobulin, were also normal.
With these results, many diagnoses were among the differential, including pericardial cysts, oncogenic cysts, lymphoma, mesotelioma, metastasic tumors, sarcoma and other mediastinal tumors such as thymoma or teratoma. Therefore, surgical resection was decided through a thoracoscopic approach. A 40 × 30 × 30 mm pearly white, pedunculated and firmly encapsulated tumor was encountered at thoracoscopy. It was near the pericardium and in close contact with the left phrenic nerve. During dissection, it was impossible to retract the phrenic nerve completely; therefore, to avoid the risk of injury, a left posterolateral thoracotomy was performed. Afterward, the entirety of the mass was removed. We dissected it from surrounding structures and completely removed it along with a small portion of the pericardium to which it was attached. The rest of the procedure was completed without complications; a bovine pericardium patch was used to repair the pericardium defect, and finally, a 28 Fr. chest drain was placed (Fig. 2).
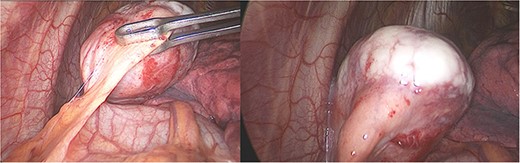
Laparoscopy, (a) hard white irregular tumor depends on the pericardium without invasion of the left phrenic nerve. (b) The tumor is adjacent to the lung but not invading it.
Pathology reported a 38 × 29 × 28 mm smooth tumor. It had a grayish external surface, few cystic areas and yellowish serous contents. The microscopic report revealed a well-defined tumor, surrounded by a thickened fibrous connective capsule with focal areas of calcification without invasion and numerous small CD5-positive lymphocytes (Fig. 3). B2 Thymoma in the mediastinum was the final diagnosis.
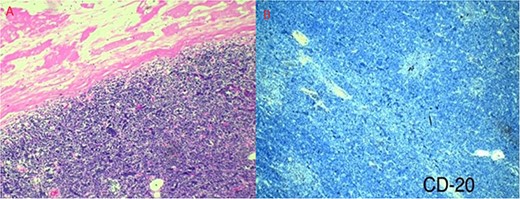
(a) Well-defined tumor formation, surrounded by a thickened fibrous connective capsule with focal areas of calcification and numerous small CD5-positive lymphocytes. (b) Immunohistochemistry shows a proliferative index of 30% with isolated positivity for CD20-positive B lymphocytes.
Her post-operative course was uneventful; the drain was removed on the third day, and she was discharged on the 5th post-operative day; since complete resection was achieved, further radiotherapy was considered but not given.
On follow-ups, she’s in close contact with oncology and is completely asymptomatic, without any tumor recurrence in over a year.
Discussion
During embryonic development, the thymus develops from the third and fourth pharyngeal ventral epithelium and gradually moves into the anterior mediastinum by Week 6 of pregnancy [1, 2]. Therefore, the majority (96%) of thymic tumors will arise in the anterior superior mediastinum [2]. When thymic tumors appear in any other place than the anterior superior mediastinum, they are called ectopic thymomas [1, 3]. These extremely rare tumors account for only 2–4% of the total thymomas; they usually appear in elderly patients and have no preferences based on gender [1, 4]. The pathological pathways for the development of these tumors are still under investigation; however, it is widely believed that if any thymus tissue remains in any other place during its embryological development, it can develop into a thymus tumor; other theories suggest that the thymoma can develop from stems cells which can explain ectopic thymoma in lungs and other places [1, 3].
Ectopic thymoma is usually located on the lungs, pleura, thyroid and pericardium but can appear in rare places such as the vertebrates or the heart [4, 5].
Pericardial thymoma is extremely rare, with few reported cases in the literature [6]. Symptoms will depend on the tumor size and invasion of surrounding structures, ranging from asymptomatic patients to fever, cough, fatigue, difficulty swallowing, chest discomfort, chest pain, palpitations, shortness of breath, hoarseness, loss of appetite, fainting pericarditis, pericardial effusion and tamponade [1, 6]. Myasthenia gravis is generally associated with thymoma but rarely related to ectopic and pericardial thymomas [3].
In our case, the patient was a completely asymptomatic young woman who was diagnosed at an early stage thanks to a chest X-ray, without which the prognosis would have changed, the tumor would have grown and more treatments would have been needed.
Imaging is needed for diagnosis and preoperative planning [1, 3, 4]. Contrast-enhanced Chest CT is the preferred imaging method for thymoma because it can show the location, size, shape, density and relationship with surrounding tissues by enhancement [5, 7]. Pericardial thymoma can be confused in the CT with a malignant teratoma or lymphoma, as they both can present as well-defined masses with heterogeneous densities [1, 6]. As it was done in our patient.
In other patients, pericardial thymomas have also presented as cysts; therefore, the differential diagnosis can be broad, including Lymphoid hyperplasia, Lung cancer, Lymphoblastic lymphoma, Teratoma, Malignant fibrous histiocytoma, Papillary thyroid carcinoma, among others [1, 4].
The definitive diagnostic is histopathological [6]. The WHO anatomopathological classification describes six morphological types (A type, AB Type, B1 type, B2 type, B3 type and C type) [8]. They are based on the histology of the thymus, since the gland has two regions: a lymphocyte-rich cortex and a medulla composed of epithelial cells [1, 2, 9].
Surgical resection is the treatment of choice, since complete resection is the best way to avoid recurrence and enhance survival [9]. Further radiotherapy is required depending on pathologic classification and completeness of resection [6].
Thanks to an early diagnosis, the patient was successfully treated, the mass was completely resected and the patient fully recovered. This particular case continues to show that pre-operative diagnosis is critical, especially in these slow-growing tumors that we usually detect when it is too late. A stroke of luck was crucial in this patient and helped her to recover completely.
Conclusion
Thymoma is a rare tumor that, unfortunately, has great clinical variability, which makes it difficult to diagnose. Because of its potential complications, the ideal treatment will be surgery; however, only a few cases will arrive at the right time. We must broaden our horizons and consider these rare pathologies when encountering a mediastinal mass. This case also proves that, as always, an adequate multidisciplinary approach is an optimal strategy for achieving the best outcomes.
Conflict of interest statement
None declared.
Funding
None declared.



