-
PDF
- Split View
-
Views
-
Cite
Cite
Mahmoud K Abd-El-Hafez, Ahmed A Romeya, Jayasheel O Eshcol, Early-to-mid onset bacterial endocarditis following implantation of Gore Cardioform Septal Occluder device, Journal of Surgical Case Reports, Volume 2025, Issue 6, June 2025, rjaf414, https://doi.org/10.1093/jscr/rjaf414
Close - Share Icon Share
Abstract
We present the unusual case of a 37-year-old female who was found to have bacterial endocarditis related to a 30 mm Gore Cardioform Septal Occluder that was placed following multiple paradoxical strokes, due to a patent PFO. Cardiothoracic Surgery was consulted and patient underwent surgical explantation with pericardial patch repair. Patient was discharged on postoperative (POD) 10 with 6 weeks of IV Cefazolin. Endocarditis following PFO closure-device implantations remains an exceedingly rare complication. In those that do develop endocarditis, the majority occur in early postop period. The first 3 months following implantation is where the subject is at greatest risk of endocarditis, owing to incomplete endothelialization of the implantable device. Late-onset endocarditis (>6 months postop) has also been documented in the literature. Thus, while current literature recommends prophylactic antibiotic administration prior to invasive surgery during the first 6 months following device implantation, prolonging this period may be indicated in select high-risk populations.
Introduction
Metanalysis of randomized control trials has consistently demonstrated that in patients with cryptogenic strokes, percutaneous surgical closure of patent foramen ovale (PFO) has resulted in superior outcomes than medical management alone. However, complications of PFO closure include thrombus formation, transient ischemic attack, pericardial tamponade, device dislocation, arrhythmias, and endocarditis. In this case presentation, we will investigate the association between endocarditis and PFO closure devices.
Case report
A 37-year-old female was transferred from referring hospital for altered mental status (AMS), fever, and hepatic encephalopathy. She was intubated on arrival for a witnessed tonic–clonic seizure. Physical exam was unremarkable except for AMS. Neurologic exam limited by sedation. Brain MRI at admission was negative. Past medical history included schizophrenia, depression, anxiety, polysubstance abuse, unspecified seizure disorder, and history of multiple cerebrovascular accident (CVA) events. Pertinent past surgical history included endovascular PFO closure 4 months earlier for cryptogenic strokes.
Baseline medications included ASA 81 mg, clopidogrel 75 mg, as well as aripiprazole, topiramate, alprazolam, and quetiapine. Pertinent abnormal lab findings included white blood count (WBC) 14.0 (4.1–11.1), sodium 147 (135–146), potassium 3.1 (3.6–5.2), lactic acid 7.3 (0.5–2.0), ammonia 138 (11–32). Urine toxicology positive for tetrahydrocannabinol (THC) only.
Prior to PFO closure, patient presented twice to the emergency room (ER), 4 months apart, for recurrent stroke-like symptoms. Brain MRI at the time demonstrated left parietal/temporal infarction. Transesophageal echocardiogram (TEE) noted a right-to-left shunt, suggestive of PFO. One month after her second visit, patient underwent an ICE-guided PFO endovascular closure procedure using a 30 mm Gore Cardioform Septal Occluder. 1 g of preoperative Vancomycin was given.
On arrival, during this hospital admission, WBC was 14.0, HR 142, Tmax 103.6. Blood cultures were positive for MSSA, and patient was started on vancomycin and ceftriaxone. Repeat blood cultures on hospital day 3 were positive for MSSA, which prompted echocardiography evaluation. Transthoracic echocardiogram was negative except for negligible tricuspid valve regurgitation. Follow-up TEE 4 days later demonstrated a mobile lesion attached to the left atrial aspect of the PFO closure device, which was concerning for vegetation (Fig. 1). Bubble study also noted a small atrial shunt. Cardiothoracic surgery was consulted and they deemed surgical explantation of the PFO device to be best-fitting.
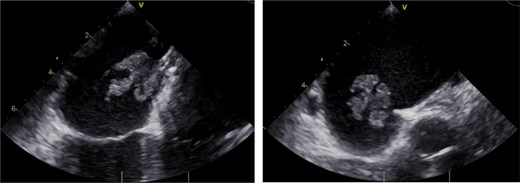
Transesophageal echocardiogram demonstrating mobile vegetation on Gore Cardioform Septal Occluder.
Patient was brought to the operating room, induced, and started on cardiopulmonary bypass following a median sternotomy and adequate aortic/bicaval cannulation. The Gore device was then accessed via an oblique right atriotomy. Under TEE guidance, the Gore device, along with the surrounding atrial septum, was excised (Fig. 2). Purulent material was seen inside the explanted vegetation. A portion of the patient’s pericardium was then harvested and used for patch repair of the large atrial septal defect that remained following explanation. The pericardial patch was anchored with running Prolene.

The surgical explanted Gore Cardioform Septal Occluder with surrounding infected atrial septum.
Patient was discharged to rehab on POD 10 with a PICC line for a 6-week regimen of IV Cefazolin per infectious disease recs (Table 1). Final pathology consistent with inflamed cardiac endomyocardium and PFO closure device with infectious vegetation.
Timeline of the events that occurred from initial ER presentation to discharge to rehab following postoperative recovery
| Week 1 – Initial presentation with right facial weakness and slurred speech. Brain MRI shows left parietal/temporal infarction, consistent with paradoxical L MCA embolic event. |
| Week 18 – Second presentation with recurrent stroke symptoms. TEE shows right-to-left shunt, suggestive of PFO. |
| Week 22 – PFO closed endovascularly, using a 30 mm Gore Cardioform Septal Occluder. |
| Week 40 – Admitted for sepsis secondary to Gore device infection. |
| Week 43 – Surgical explantation of infected Gore closure device with pericardial patch repair of the surgically created atrial septal defect. |
| Week 45 – Discharged on POD 10 to rehab with a 6-week course of Cefazolin IV. |
| Week 1 – Initial presentation with right facial weakness and slurred speech. Brain MRI shows left parietal/temporal infarction, consistent with paradoxical L MCA embolic event. |
| Week 18 – Second presentation with recurrent stroke symptoms. TEE shows right-to-left shunt, suggestive of PFO. |
| Week 22 – PFO closed endovascularly, using a 30 mm Gore Cardioform Septal Occluder. |
| Week 40 – Admitted for sepsis secondary to Gore device infection. |
| Week 43 – Surgical explantation of infected Gore closure device with pericardial patch repair of the surgically created atrial septal defect. |
| Week 45 – Discharged on POD 10 to rehab with a 6-week course of Cefazolin IV. |
Timeline of the events that occurred from initial ER presentation to discharge to rehab following postoperative recovery
| Week 1 – Initial presentation with right facial weakness and slurred speech. Brain MRI shows left parietal/temporal infarction, consistent with paradoxical L MCA embolic event. |
| Week 18 – Second presentation with recurrent stroke symptoms. TEE shows right-to-left shunt, suggestive of PFO. |
| Week 22 – PFO closed endovascularly, using a 30 mm Gore Cardioform Septal Occluder. |
| Week 40 – Admitted for sepsis secondary to Gore device infection. |
| Week 43 – Surgical explantation of infected Gore closure device with pericardial patch repair of the surgically created atrial septal defect. |
| Week 45 – Discharged on POD 10 to rehab with a 6-week course of Cefazolin IV. |
| Week 1 – Initial presentation with right facial weakness and slurred speech. Brain MRI shows left parietal/temporal infarction, consistent with paradoxical L MCA embolic event. |
| Week 18 – Second presentation with recurrent stroke symptoms. TEE shows right-to-left shunt, suggestive of PFO. |
| Week 22 – PFO closed endovascularly, using a 30 mm Gore Cardioform Septal Occluder. |
| Week 40 – Admitted for sepsis secondary to Gore device infection. |
| Week 43 – Surgical explantation of infected Gore closure device with pericardial patch repair of the surgically created atrial septal defect. |
| Week 45 – Discharged on POD 10 to rehab with a 6-week course of Cefazolin IV. |
Discussion
In this report, we present the case of an early-to-mid-onset PFO closure-device-associated endocarditis in an otherwise healthy patient. Metanalysis of randomized control trials has consistently demonstrated that percutaneous surgical closure of PFO has resulted in superior outcomes than medical management alone [1]. However, complications of PFO closure include thrombus formation, transient ischemic attack, pericardial tamponade, device dislocation, arrhythmias, and endocarditis [1].
Endocarditis following PFO closure-device implantations remains an exceedingly rare complication. Management revolves around minimizing risk factors and adequate pre- and postoperative antibiotic utilization. This patient had no risk factors (immunosuppression, poorly controlled comorbidities, etc.) or signs of infection (skin rash, UTI, dental infections) at the time of her initial percutaneous device placement. Given her amoxicillin and clindamycin allergies, vancomycin was given preoperatively. Current guidelines recommend 15–20 mg/kg. Given her preoperative weight of 72 kg, patient received adequate coverage with 1 g of vancomycin. However, one notable finding was the time between antibiotic administration and percutaneous entry. The access time during her PFO device placement was only 9 min following administration of vancomycin. CDC guidelines recommend waiting 60–120 min prior to incision or percutaneous entry to minimize risk of infection. In a 2020 retrospective cohort study, administering vancomycin infusion <15 min prior to incision (as in this patient) resulted in an odds ratio (OR) of 4.23 for postoperative SSI, compared to the recommended 60–120 min window (OR 0.24) [2].
In those that go on to develop endocarditis, the vast majority occur in early postop period. In a 2016 case series, 22 of the 22 patients who went on to develop endocarditis did so at or before the 1-year mark [3]. Late-onset endocarditis, defined as endocarditis developing >6 months postoperatively, has also been documented in the literature. There are at least four documented incidences of late-onset endocarditis [4]. The latest of which was a 15-month postop and was treated surgically. The exact reasons for late device infection are poorly understood. One hypothesis is that protruding/exposed titanium wiring could have served as the initial nidus for infection, with subsequent erosion through the endothelial layers by infectious biofilms and vegetation [4].
The first 3 months following device implantation is where the subject is at the greatest risk of endocarditis, owing to incomplete endothelialization of the implantable device. Several animal studies have demonstrated a strong inverse relationship between the risk of endocarditis and the total duration of time since surgery [5]. However, even at 5-month postop, human autopsy findings have shown that there is still almost no endothelialization on the surface of metallic mesh, despite contrary evidence seen on echocardiography at that time [5]. Thus, prolonging the prophylactic antibiotic window for high-risk patients may be a key component for preventing late-onset endocarditis following PFO device implantations.
There are several elements that comprise the novelty of this case report. An otherwise healthy 37-year-old patient presented to the ER multiple times for recurrent strokes. She was not provided with the proper cardiac workup until her last visitation, allowing her PFO to go undiagnosed and untreated for 4 months. Following this incident, a PFO or ASD should be high on the reader’s differential whenever they encounter a stroke in an otherwise unusual patient population. The lack of risk factors was also unusual, as this patient lacked any medical comorbidities or evidence of ongoing or recent systemic infection at the time of Gore device placement. Third, most documented cases of endocarditis following endovascular placement of occluding devices occur in the early period, defined by <3 months, due to the ongoing process of endothelialization coating the device. Our case demonstrates that the ongoing risk of endocarditis outside this window is not negligible, which challenges the dogma that prophylactic antibiotics prior to invasive procedures should only be administered in the first 6 months following placement. Lastly, the majority of endocarditis cases following occluding device placement, which is documented in the literature, are managed conservatively, as open cardiovascular intervention is often deemed too high risk. The reliance on long-term antibiotic administration and the process of reendothelialization is not always enough. Knowing the surgical techniques available to manage these rare complications, when they do arise, is critical for the successful management of this patient population.
Acknowledgements
There are no personal financial disclosures, non-financial support, or conflict of interest to be acknowledged during the making of this case report. This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities. No external funding was obtained for the completion of this case report.
Conflict of interest statement
No other financial disclosures or conflict of interest.
Funding
This research was supported (in whole or in part) by HCA Healthcare and/or an HCA Healthcare-affiliated entity. The views expressed in this publication represent those of the author(s) and do not necessarily represent the official views of HCA Healthcare or any of its affiliated entities.



