-
PDF
- Split View
-
Views
-
Cite
Cite
Zehra Özgen, Bilge Çataloğlu, Dilek Altun, Emre Sahillioğlu, Merve Şeker, Sıla Karaman, Yasemin Kosdak, Sumru Gezgin, Deniz Kahraman, Yeşim Öztürk, Filiz Saçan, Erdem Yildiz, Alp Dinçer, Meltem Saraç, Cem Akbal, Agageldi Annayev, Agop Çıtak, Didem Büyüktaş, İlkay Değerli, Müge Kocak, Bahattin Tanrıkulu, Burak Tander, Hakan Ağır, Memet Özek, Erdi Dirilen, Kutalp Kurt, Mehtap Seluck, Anesthetic planning and management for successful separation surgery of pygopagus conjoined twins: a multidisciplinary approach, Journal of Surgical Case Reports, Volume 2025, Issue 6, June 2025, rjaf320, https://doi.org/10.1093/jscr/rjaf320
Close - Share Icon Share
Abstract
Conjoined twins are rare and present significant challenges for surgical and anesthetic teams. Pygopagus twins, fused at the pelvis and lower spine, require meticulous planning, and multidisciplinary collaboration. This report describes the elective separation of pygopagus twin girls, involving advanced imaging, 3D anatomical modeling, and simulation-based preparation. A color-coded system differentiated anesthesia teams, equipment, and monitors, ensuring clarity during the 27-h procedure. Anesthesia was maintained with sevoflurane and remifentanil, with individualized fluid and hemodynamic management. Surgical steps included separation of shared spinal, rectal, and urogenital structures, followed by soft tissue reconstruction. The surgery was completed without major complications. Both twins were extubated within seven days, recovered well in the pediatric ICU, and were discharged on postoperative day 56 without neurological deficits. This case highlights the value of thorough planning, advanced imaging, and a structured multidisciplinary approach. Color-coded systems and simulations enhanced coordination, reduced risks, and improved outcomes.
Introduction
Conjoined twins (CJT) are a rare congenital anomaly, occurring in approximately one in 49 000–189 000 live births, with higher incidence in Southwest Asia and Africa. Despite advances in medical care, the mortality rate remains high; ⁓60% are stillborn, and 35% die within the first 24 h [1, 2]. Only 5%–25% survive long enough to undergo separation surgery. Historical records date back to 1000 B.C., highlighting the longstanding medical challenge [3]. CJT are classified by fusion site, including thoracopagus, craniopagus, omphalopagus, ischiopagus, rachipagus, cephalopagus, and pygopagus; the latter comprising 19% of cases and associated with the lowest separation mortality [4, 5]. Successful separation depends on multiple factors such as attachment site, age, comorbidities, and extent of shared organs [3, 6, 7].
Multidisciplinary planning, using magnetic resonance imaging (MRI), CT with 3D reconstruction, angiography, and ultrasound, is essential to map shared anatomy and guide intervention [5, 8–10]. Simulation-based rehearsals, 3D modeling, and color-coded systems optimize team coordination. Anesthesia involves careful preoperative preparation, imaging sedation, safe positioning, ventilation, hemodynamic stability, and complication management [3–6, 8]. This case report details anesthesia management for pygopagus twin separation, highlighting advanced imaging, 3D modeling, simulation, and a color-coded system for team coordination. Neuro-monitoring and tailored fluid management ensured stability, offering key insights for similar complex pediatric surgeries.
Case presentation
History
Nine-month-old Nigerian pygopagus conjoined twin girls were admitted for planned separation. Both were exclusively breastfed, developmentally normal, and showed no abnormalities in vital organ functions (Fig. 1).

Preoperative investigations
Weighing 15 kg combined, the twins were joined at the sacral region, with partial fusion of hips. Each had a single kidney and separate bladders, with shared ureteral and anal openings, and two vaginal orifices. Twin A was slightly larger and calmer. No spinal cross-connections were noted. Investigations included MRI, angiography, and endoscopic procedures. Imaging revealed fused sacral vertebrae, shared dural sac, tethered cords, and convergence of two rectums into a single anal canal. Vascular anastomoses existed between the internal iliac branches (Figs. 2 and 3).
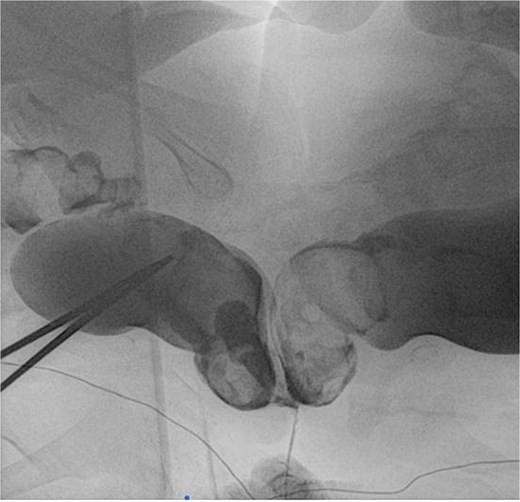
Rectal contrast filled two separate rectums fused distally to form a single anal canal.
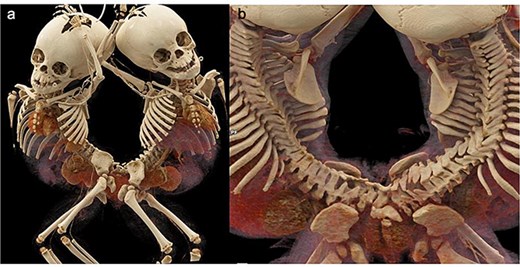
3D CT demonstrations of pygopagus twins. (a) Anterior and posterior views. Scoliosis and multiple segmentation and fusion anomalies of several vertebrae and sacrums of both babies, more so in the baby on the right, (b) distal sacral and coccygeal fusion.
Anesthesia and 3D modeling
MRI and angiography were done under sedation using sevoflurane and propofol. Each twin had a dedicated anesthetic team. Atropine testing ruled out cross-circulation. Shared CSF circulation and vascular overlap were confirmed (Fig. 4).
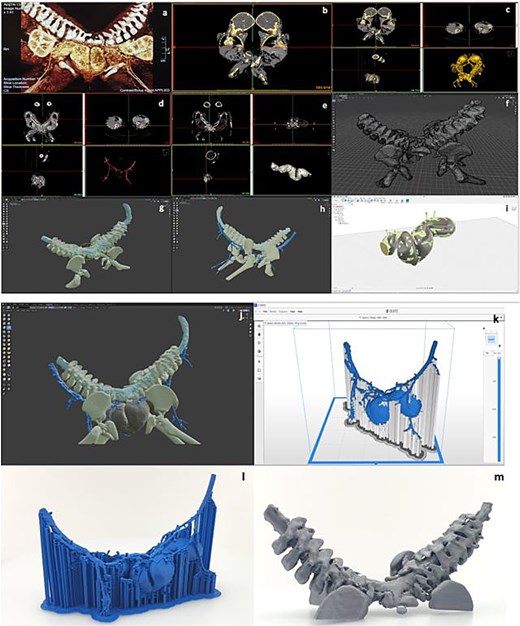
Steps for 3D model preparation: (a) examination of the radiological images, (b) choosing the bony structures at the MRI image, (c) obtaining the 3D model of the bony structure from the MRI image, (d) obtaining the 3D model of the vascular structures from the MRI, (e) obtaining the 3D model of the bowel from the MRI image, (f) construction of the 3D model of the bony structure, (g) arrangement and positioning of the 3D model of the spinal cord, (h) arrangement and positioning of the 3D model of the vascular network, (i) remodeling of the intestine based on the reference model obtained from radiological images, (j) arrangement and positioning of the 3D model of the bowel, (k) slice of the intestinal and vascular network model in Zortrax Z-suite software, (l) 3D printing of the intestinal and vascular network model.
A 3D anatomical model was created using MRI/magnetic resonance angiography (MRA) data for surgical planning. The model included the spine, spinal cord, hips, intestines, and vessels. It was refined with specialized software and printed using multiple filaments, aiding preoperative simulations (Fig. 4).
Diagnostic procedures and placement of expanders
A full-team simulation preceded diagnostics. The twins underwent endoscopic evaluation under general anesthesia in a hybrid OT. Two urethras and bladders were noted; Twin A lacked an external urethral opening. No complications occurred during the 4-h procedure (Fig. 5).
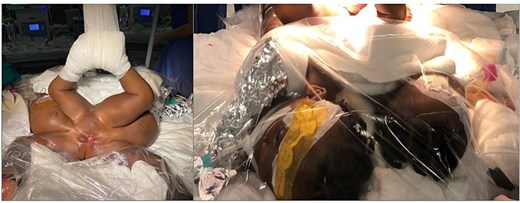
(Left) horizontal placement and the special litotomy position of the infants; (right) the twins were monitored, intubated and heat loss was prevented during the long diagnostic procedures.
Tissue expanders were inserted in the gluteal region to aid closure postseparation. Twin B developed local inflammation requiring replacement. Colostomy was performed 4 months later. Twin A experienced post-op sepsis and colostomy eventration, delaying separation.
Separation surgery
One month after colostomy, the surgical plan was finalized following discussion among the anesthetic, surgical, and intensive care team. The twins were prepared in the Pediatric Intensive Care Unit (PICU). The central and peripheral IV lines were placed under sedation the day before the surgery. The twins were induced with sevoflurane (4%–6% in O2/Air) at 2 L/min, each by separate anesthesia teams each with its own color-coded monitors, drugs, anesthesia machines, breathing systems, syringes, and anesthetic and blood gas charts, as previously practiced.
Radial artery cannulation was performed on both twins to monitor invasive blood pressure, arterial blood gas levels and perform serial blood investigations during the lengthy procedure. Vital parameters such as HR, BP, SpO2, ETCO2, esophageal body temperature, BIS and regional cerebral oxygenation via near- infrared spectroscopy (NIRS) were monitored. Neuro-monitoring included somatosensory and motor-evoked potentials (SEP and MEP). As practiced during simulations, the twins were placed in prone U-shaped position (Fig. 6) for neurosurgical separation of the common spinal and dural structures, which took 5.5 h. A surgical cover was placed beneath the infants to facilitate repositioning during subsequent stages of surgery. After separation of the spinal structures and closure of dural sac under close neuro-monitorization, the twins were positioned supine for the removal of the expanders. Under high-resolution microscopy, careful dissection was performed to separate the neural elements. Continuous intraoperative neuromonitoring, including somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEPs), was employed to preserve neurological integrity. No significant signal drop was observed throughout the procedure. Following separation, the individual spinal cords were reconstructed and dural closure was achieved using synthetic grafts to maintain CSF integrity and prevent tethering. Reconstruction of the rectal and anal structures followed the ligation of shared circulation points around rectum identified via angiography.
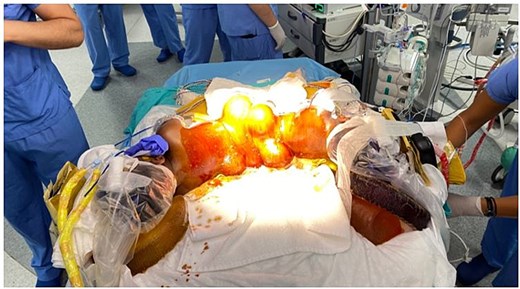
Postoperative PICU care
Both twins were ventilated post-op. Fluid and blood product administration in the twins are described in Table 1. Twin A developed TRALI, resolved by POD 2. A CSF leak on POD 5 required surgical repair. Both were extubated by POD 7. Upon reuniting, they displayed emotional bonding (“twin phantom syndrome”). Twin A later had a fluid collection that resolved with antibiotics. Both were discharged on POD 56.
| Twin A . | Twin B . |
|---|---|
| 1250 mL Crystalloid | 1180 mL Crystalloid |
| 625 mL FFP | 700 mL FFP |
| 700 mL ES | 750 mL ES |
| 30 mL Albumin (20%) | 30 mL Albumin (20%) |
| Twin A . | Twin B . |
|---|---|
| 1250 mL Crystalloid | 1180 mL Crystalloid |
| 625 mL FFP | 700 mL FFP |
| 700 mL ES | 750 mL ES |
| 30 mL Albumin (20%) | 30 mL Albumin (20%) |
| Twin A . | Twin B . |
|---|---|
| 1250 mL Crystalloid | 1180 mL Crystalloid |
| 625 mL FFP | 700 mL FFP |
| 700 mL ES | 750 mL ES |
| 30 mL Albumin (20%) | 30 mL Albumin (20%) |
| Twin A . | Twin B . |
|---|---|
| 1250 mL Crystalloid | 1180 mL Crystalloid |
| 625 mL FFP | 700 mL FFP |
| 700 mL ES | 750 mL ES |
| 30 mL Albumin (20%) | 30 mL Albumin (20%) |
Discussion
This case reports the successful elective separation of pygopagus twins using advanced imaging, 3D modeling, and multidisciplinary simulation. Challenges like a shared spinal cord and vessels were managed with neuromonitoring, dual anesthesia teams, and color-coded coordination for safety. Intraoperative neuromonitoring was crucial during dissection of the conjoined spinal cord, preserving SSEPs, and MEPs to ensure functional integrity. No postoperative neurological deficits were observed, emphasizing the importance of neuro-monitoring in reducing injury risk, particularly in complex U-shaped spinal fusion cases.
Unlike high-risk emergency separations, elective timing enabled thorough counseling, nutritional optimization, and risk mitigation. Ethical oversight and informed parental consent ensured transparency. This case highlights the value of early planning, cross-specialty collaboration, and technology in improving CJT outcomes, contributing to the limited but growing record of successful pygopagus separations in resource-limited settings.
Acknowledgements
We acknowledge the parents of the patients for their cooperation.
Author contributions
Zehra Serpil Ustalar Özgen: Conceptualization of the study, coordination of the surgical team, and drafting of the manuscript; Bilge Şentürk Çataloğlu: Provided expertise in anesthetic management and contributed to manuscript preparation; Dilek Altun: Participated in preoperative diagnostic simulations and postoperative monitoring; Emre Sahillioğlu: Contributed to the design and execution of surgical procedures and data interpretation; Merve Şeker: Assisted with preoperative diagnostic procedures and participated in intraoperative neuro-monitoring; Sıla Karaman: Managed perioperative nursing care and participated in simulation-based preparations; Yasemin Kosdak: Contributed to the preparation and execution of preoperative simulation exercises; Sumru Gezgin: Assisted in radiological diagnostics and preoperative planning; Deniz Kahraman: Participated in surgical procedure execution and assisted in postoperative care; Yeşim Öztürk: Contributed to patient monitoring during anesthetic management and manuscript writing; Filiz Saçan: Assisted in perioperative fluid management and blood transfusion protocols; Erdem Yildiz: Played a role in intraoperative monitoring and surgical support; Alp Dinçer: Provided expertise in radiological imaging and analysis; Meltem Saraç: Participated in preoperative diagnostic and intraoperative monitoring; Cem Akbal: Contributed to surgical planning and operative execution; Agageldi Annayev: Participated in postoperative follow-up and data collection; Agop Çıtak: Supervised the pediatric care team and provided postoperative clinical management; Didem Büyüktaş Aytac: Contributed to diagnostic and interventional procedures and manuscript revision; İlkay Değerli: Provided expertise in neurosurgical planning and intraoperative procedures; Müge Kocak: Assisted in coordinating multidisciplinary team efforts and managing patient care logistics; Bahattin Tanrıkulu: Participated in preoperative planning and execution of surgical procedures; Burak Tander: Contributed to operative strategy formulation and patient follow-up; Hakan Ağır: Provided support in surgical execution and intraoperative management; Memet Özek: Supervised the neurosurgical procedures and contributed to manuscript preparation; Erdi Görkem Dirilen: Played a key role in the design and implementation of surgical interventions; Kutalp Kurt: Coordinated diagnostic evaluations and contributed to the overall study design; Mehtap Selcuk: Provided support in surgical execution and intraoperative management.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
All the data generated from the study is available in the manuscript.
Consent for publication
Informed consent has been obtained from the parents of the patients.
Ethics approval and consent to participate
The study has been conducted in accordance to the declaration of Helsinki and ethical approval was obtained from the Institutional Review Board (IRB) of Acıbadem Üniversitesi. Informed consent to participate was obtained from the parents of the patients.



