-
PDF
- Split View
-
Views
-
Cite
Cite
Alexander Pohlman, Mohammad Nizamuddin, Fritzie S Albarillo, Zaid M Abdelsattar, Lung resection from wedge to pneumonectomy as surgical options for pulmonary mucormycosis, Journal of Surgical Case Reports, Volume 2024, Issue 11, November 2024, rjae753, https://doi.org/10.1093/jscr/rjae753
Close - Share Icon Share
Abstract
Pulmonary mucormycosis (PM) is a rare and life-threatening condition, most prevalent in immunocompromised patients. Early signs and symptoms are often nonspecific. A high index of suspicion in at risk patients should prompt early infectious work-up, including bronchoscopy, followed by aggressive antifungal therapy and early surgical resection when indicated. We demonstrate these core tenants of diagnosis and management of PM via two patient presentations, the first involving a kidney transplant recipient who presented with a mild cough, found to have a lung lesion with rapid growth over a few weeks; the second involving a patient with acute lymphoblastic leukemia who presented with hemoptysis and imaging revealing a 5 cm perihilar mass obliterating the left pulmonary artery. Both patients were managed with aggressive surgical therapy.
Introduction
Pulmonary mucormycosis (PM) is a life-threatening condition that develops after the inhalation of fungal spores into the patient’s lungs. The disease is most commonly seen in patients with immune defects, such as those secondary to immunosuppressant medications and immune-related diseases [1]. PM typically progresses rapidly into pneumonia and is characterized by its invasiveness and destruction of bronchi, lung parenchyma, and vascular structures. Early PM symptoms are non-specific and include fever, dyspnea, cough, and chest pain. More advanced symptoms, may include hemoptysis, Pancoast syndrome, Horner’s syndrome, and can rapidly progress to respiratory distress and death [2]. We present two cases of PM in a patient on immunosuppressants and in another patient with acute lymphoblastic leukemia.
Case series
Case 1
A 63-year-old male with a history of heart failure, hypertension, diabetes, and recent renal transplant 8 months prior, currently on immunosuppressant medication, was referred to the emergency department from his primary care physician due to several days of chest pain, fatigue, vomiting, and diarrhea. On arrival, he was afebrile and was adequately oxygenating on room air. Cardiac work-up revealed normal troponin and B-type natriuretic peptide levels. Infectious work-up revealed COVID-19 positivity and chest X-ray revealed a nodular opacity in the left upper lobe (LUL) of the lung.
After 2 days, a follow-up chest X-ray showed a more prominent-appearing lung mass, prompting the ordering of a chest computed tomography (CT) scan. The CT scan revealed a focal mass-like consolidation measuring 2.4 × 2.2 cm in the LUL (Fig. 1A). Further infectious work-up including fungal antigen markers for Aspergillus, histoplasmosis, and blastomycosis were negative. His COVID-19 symptoms improved, so he was discharged home with close follow-up.
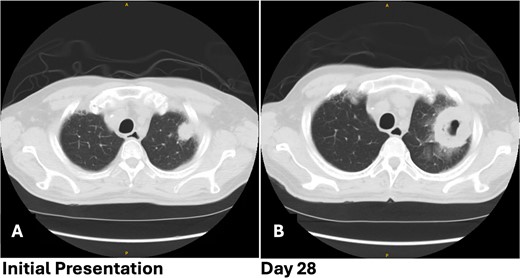
Patient 1 pre-operative images showing rapid progression of disease. (A) Initial CT scan with a 2.4 cm LUL pulmonary nodule. (B) Repeat CT scan 28 days later showing rapid growth and cavitation of the mass, now measuring 7.1 cm.
The patient returned with hypotension, weakness, and a presyncopal episode secondary to Clostridium difficile infection and dehydration. Chest imaging showed further growth of the LUL mass now up to 7.1 cm with cavitation from 4 weeks earlier (Fig. 1B). A flexible bronchoscopy was performed and samples sent for pathology and microbiology. This revealed extensive tissue necrosis and fungal hyphae consistent with mucormycosis.
Due to concerns of nephrotoxicity from Amphotericin B in a recent kidney transplant recipient, the patient was started on isavuconazole. Thoracic surgery evaluated the patient and recommended early surgical resection. The operation was started as a video-assisted thoracoscopic surgery, but due to dense adhesions a posterolateral thoracotomy was needed. The mass was in the apex of the LUL with extensive adhesions to the apex. There was healthy lung in the LUL inferior to the mass, therefore a large wedge resection with the stapler was performed. The dense vascular adhesions at the apex required extra pleural dissection. The patient was extubated in the operating room and recovered on the floor after the procedure. Following the surgery, the patient continued treatment with isavuconazole for a minimum of one year with the possibility for indefinite therapy. He remains on isavuconazole and has shown no recurrence of mucormycosis, infectious complications, or mortality 18 months post-operatively.
Case 2
A 60-year-old woman with acute lymphoblastic leukemia recently started on chemotherapy presented to the emergency department with a dry cough that later progressed to a productive cough with green sputum. Exam was largely unremarkable as patient was afebrile and had normal lung auscultation. Initial infectious work-up, including bacterial, fungal and mycobacterial cultures, fungal serum and urine antigens, and COVID-19 testing were negative. Given a negative infectious work-up, a CT chest, abdomen, and pelvis was obtained revealing a 5 cm perihilar mass causing complete obliteration of the left pulmonary artery and minimal-to-no perfusion to the left lung (Fig. 2).
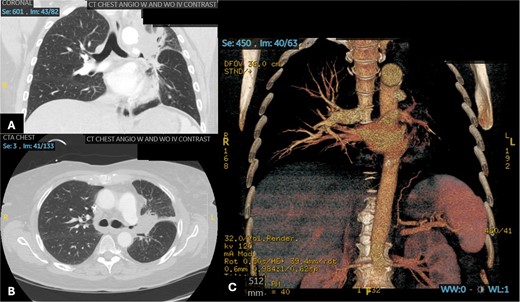
Patient 2 pre-operative images. (A) Coronal section of CT angiogram showing LUL mass abutting the aortic arch and invading the left main pulmonary artery and left mainstem bronchus. (B) Axial CTA showing complete occlusion of the left pulmonary artery. (C) 3D reconstruction of CTA showing complete occlusion of the left pulmonary artery and lack of opacification of any pulmonary artery branches and pulmonary oligemia.
A bronchoscopy was completed for biopsy of the lesion and revealed angioinvasive mucormycosis. The patient was immediately started on amphotericin and isavuconazole and evaluated by thoracic surgery for possible surgical resection. Given the perihilar location involving the pulmonary artery, it was likely that a pneumonectomy would be required. Pulmonary function tests (PFTs) were completed and showed an FEV1 of 54% predicted and diffusing capacity of the lungs for carbon monoxide (DLCO) of 57% predicted. Although these PFTs alone suggest that a pneumonectomy may not be tolerated; it is important to note that on the CT scan there was no perfusion to the left lung. A V/Q scan confirmed absent perfusion to the left lung given the invasion of the pulmonary artery with only 3.4% of perfusion reaching the left lung. Therefore, a pneumonectomy would not change PFTs significantly.
The patient underwent a muscle sparing left thoracotomy, extrapleural intrapericardial left pneumonectomy, and serratus anterior muscle flap to the bronchial stump. The operation was challenging as the fungal mass was densely adherent to the chest wall and all hilar structures, requiring isolation of the pulmonary artery intrapericardially. A serratus anterior muscle flap was then placed over the bronchial stump. Estimated blood loss was 1000 mL intraoperatively. The patient did well post-operatively, only required transfusion of 1 unit of packed red blood cells on postoperative day 3 and was discharged on postoperative day 5. She was doing well without dyspnea, and free of fungal disease on her most recent follow-up 18 months after her operation. Follow-up chest CT scan can be seen in Fig. 3.
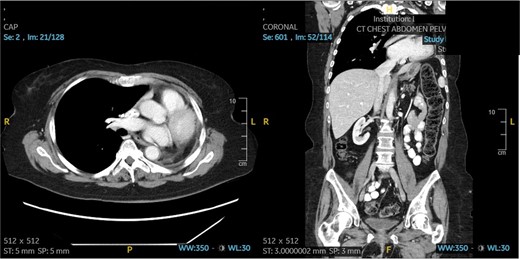
Patient 2 post-operative CT scan showing complete resection of disease and expected post-operative anatomical changes, including leftward deviation of mediastinum and hemidiaphragmatic elevation.
Discussion
We present two cases of PM involving rapid growth of a cavitary lung lesion from ~2 to 7 cm in 4 weeks, and an aggressive angioinvasive mass occluding the left main pulmonary artery. Early and aggressive surgical intervention is highlighted in both cases after confirming the diagnosis through analysis of pathological and microbiology specimens obtained via bronchoscopy.
As symptoms and radiological characteristics are nonspecific, early diagnosis with bronchoscopy and tissue biopsy with cultures are needed in high-risk patients [1, 3, 4]. More specifically, histopathological identification of tissue necrosis and neutrophilic infiltration with broad, non-septate ribbon-like hyphae with right angled branching in tissue biopsy confirms the diagnosis. Less common histological features include bronchial invasion, pneumonia, lung abscesses, and granulomatous pneumonitis [5].
Early diagnosis is critical to treatment of PM, considering its rapid progression and poor survival outcomes, which is complicated by its difficulty in identification. Mucorales, the fungi that causes PM generally grows in decayed organic matter such as fruit, soil, or manure, and can grow in both aerobic as well as anaerobic conditions; however, cultures often result negative as the organism does not grow readily, which will complicate making the diagnosis. There are currently no existing serological or immunologic antibody tests to identify PM. Because PM is often seen with bacterial pneumonia, the categorical diagnosis of PM as a primarily fungal infection is often delayed for more generic pneumonia specific treatment in the initial treatment course [3].
One of the difficulties of PM is the fact that there are very few differentiators between PM and other more common and prominent fungal infections, such as Aspergillus on imaging. Recently, polymerase chain reaction (PCR) methods have been used to help differentiate between Aspergillus and Mucor [6, 7]. PM has a significantly poorer prognosis and outcome compared to aspergillosis, partially due to delayed diagnosis and ineffective antifungals. PM has an overall mortality of 76% which goes up to 95% in patients with extrathoracic dissemination [1, 2, 8]. Untreated mucormycosis rarely has shown survival beyond 2 weeks as shown in the literature, with less than 50% of patients being diagnosed premortem [9, 10]. We were not able to find in existing literature any mention of growth rates of pulmonary lesions associated with mucormycosis. However, the doubling time of the lesion on imaging of 4 weeks in our case highlights its aggressive nature. Although such rapid growth is possible with aggressive malignancy, it should raise suspicion for invasive fungal infections.
The current gold standard treatment for PM is early surgical resection and amphotericin B [11]. Medical management alone has been shown to be far inferior than when combined with surgery. One study revealed a mortality rate of 50% with medical therapy alone vs 9.4% when surgery is part of the treatment [12]. However, amphotericin B has been shown to have dose-limiting nephrotoxicity, and thus is not suitable for renal transplant patients, which creates a treatment conundrum given that this is precisely one of the populations that is most susceptible to Mucormycosis [13]. The Valuation of Isavuconazole Treatment in Aspergillosis and other Life-threatening fungal infections (VITAL) and ISAvuconazole treatment for fungal infections in Solid Organ Transplant (ISASOT) trials, showed that isavuconazole is equally effective in the treatment of invasive fungal disease compared to amphotericin and is safe in solid organ transplant recipients, making this a reasonable alternative for renal transplant patients [14, 15]. Oral Posaconazole is also recommended situationally but has not shown to be effective without surgery, again demonstrating the utmost importance of early surgical intervention [1, 16–19]. However, surgical interventions are shown to be associated with lower mortality rates in PM patients when combined with proper medical therapy, so co-management with antifungals is still crucial [1, 20–23]. Additionally, recurrence rates are higher in patients that do not have bimodal treatment regimens involving both surgery and medical interventions [24]. It is important to note however, that not all patients may be rescued with surgery. Patients with bilateral disease are generally deemed not surgical candidates.
Conclusion
These two cases of rapidly progressive and aggressive PM infections highlight the critical importance of early diagnosis and surgical treatment. PM often presents with non-specific symptoms and radiological findings, leading to delayed identification. These cases display the challenges in differentiating PM from other fungal infections, like aspergillosis, and the necessity for specific diagnostic methods, such as endobronchial biopsy and the new development of PCR. The mortality rate for PM is dangerously high, emphasizing the urgency of timely intervention. Treatment options, including surgical resection and alternative antifungal therapies, must be carefully considered, particularly in immunocompromised patients with renal or transplant concerns. This case underscores the need for heightened awareness and prompts action in managing PM, as early diagnosis and early surgical resection remain the keys to improving survival outcomes.
Conflict of interest statement
None declared.
Funding
None declared.



