-
PDF
- Split View
-
Views
-
Cite
Cite
Joel Ern Zher Chan, Zhihong Kuah, Shantanu Bhattacharjya, Santosh Antony Olakkengil, Recurrent thromboses and major vessel compressions in autosomal dominant polycystic kidney disease, Journal of Surgical Case Reports, Volume 2022, Issue 2, February 2022, rjac012, https://doi.org/10.1093/jscr/rjac012
Close - Share Icon Share
Abstract
A 41-year-old man with autosomal dominant polycystic kidney disease (ADPKD), who had multiple previous unprovoked thrombotic events and without a known coagulopathic disorder, presented with symptomatic extensive thrombus distal to the compression site of the left common iliac vein by a dominant cyst in the left inferior renal pole. This was managed with inferior vena cava filter insertion, left nephrectomy and warfarinization. Later, there was inferior vena cava compression by the right polycystic kidney, leading to elective right nephrectomy. Post-renal transplantation, he had further episodes of partial dialysis access stenosis and extensive thromboses in the left deep and right superficial venous systems of the lower limbs despite absence of extrinsic compression. This represents the first report of recurrent mass effect and thromboembolic events in ADPKD, both before and after nephrectomy and anticoagulation. The potential increased thromboembolic risks among patients with ADPKD warrant further investigation.
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney condition, affecting 12 million people globally [1]. Around 70% of ADPKD patients become dependent on renal replacement therapy, and many experience acute and chronic pain, cyst infections, rupture and hemorrhage and hypertension [1]. ADPKD patients have an increased risk of developing intracranial cerebral aneurysms and polycystic liver disease [1, 2], whereas evidence on renal cell carcinoma is conflicting [3–5].
Mass effect and compression of other organs had been reported in ADPKD, although disproportionately as a result of liver cysts rather than kidney cysts. Few cases of thromboembolic events (TEs) as a result of major vessel compression had been reported [6–10], although inferior vena cava (IVC) compression of unclear significance and post-transplantation TE risks appear to be common among ADPKD patients [11, 12].
We report a case of an ADPKD patient who was repeatedly found to have major vessel compression by dominant renal cysts and recurrent TEs, both before and after nephrectomy and anticoagulation therapy.
CASE REPORT
A 41-year-old man on hemodialysis for end-stage renal failure from maternally inherited ADPKD presented with a 2-week history of left calf pain and progressively increasing peripheral edema. He was otherwise asymptomatic with no provoking factors. His past medical history was significant for multiple TEs, including bilateral unprovoked upper limb deep vein thromboses (DVTs) and several episodes of arteriovenous fistula thromboses requiring thrombectomy and/or fistuloplasty. He also had hypertension and hypercholesterolaemia.
Physical examination revealed above-knee swelling and palpable irregular abdominal mass bilaterally, the latter of which is consistent with ADPKD. His laboratory investigations were unremarkable with platelet count of 256 × 109/l, normal prothrombin time, activated partial thromboplastin time and negative anti-cardiolipin antibodies and beta-2 glycoprotein 1 antibodies. Previous thrombosis evaluation, following an incidental finding of right upper limb DVT, was normal for antithrombin III activity, protein C activity, protein S levels, Factors II and V and anti-cardiolipin antibodies.
Ultrasound (US) and subsequent computed tomography (CT) of the abdomen and pelvis revealed compression of the left common iliac vein by a dominant cyst of the left polycystic kidney (Fig. 1), with extensive non-occlusive thrombus through to the left calf. There were also multiple hepatic cysts in both liver lobes, and the duodenal segments D1 and D2 had been displaced to the left, but there was no evidence to suggest bowel obstruction.
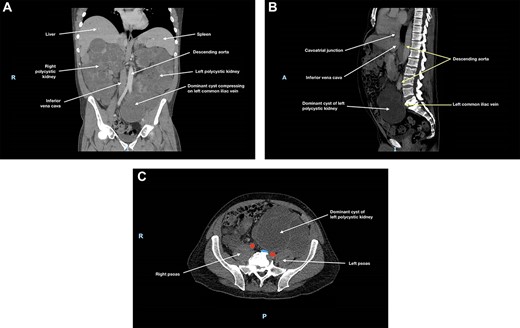
(A) Coronal, (B) sagittal and (C) axial views of the patient’s CT scan of his abdomen and pelvis at initial presentation; this showed enlarged polycystic kidneys, with the left kidney extending into the patient’s pelvis; there was compression of the left common iliac vein by a dominant cyst in the left polycystic kidney against the patient’s sacrum; in (c), the left common iliac vein is depicted in blue and the common iliac arteries are depicted in red; hepatic cysts and duodenal effacement are not visible in the selected planes; R, right; I, inferior; A, anterior; P, posterior.
An emergency left nephrectomy was performed following IVC filter insertion. The kidney measured 350 × 200 × 200 mm and weighed 5530 g; no malignancy was identified. A subsequent CT angiogram, undertaken for consideration of thrombectomy, incidentally identified compression of the IVC by the right polycystic kidney, with near-complete effacement.
The patient was commenced on 6-month warfarin therapy and the IVC filter was retrieved 6 weeks post-insertion. A catheter venogram conducted prior to IVC filter removal revealed patent right-sided veins with no thrombus; this was confirmed on subsequent duplex US. Intracranial aneurysm was ruled out.
At 3-month follow-up, CT angiogram showed persistent IVC compression by the right polycystic kidney, with near-complete effacement (Fig. 2). There was no thrombus distal to this compression and the patient was asymptomatic. May-Thurner syndrome was ruled out. As the right kidney had been found to cause IVC compression and to extend into the pelvis, an elective right nephrectomy was performed to prevent further thromboses in the right-sided deep venous systems and to create space for subsequent transplantation. The right kidney measured 270 × 170 × 150 mm and weighed 3098 g. There was extensive cystic alteration with fibrosis and tubular atrophy; no malignancy was identified.
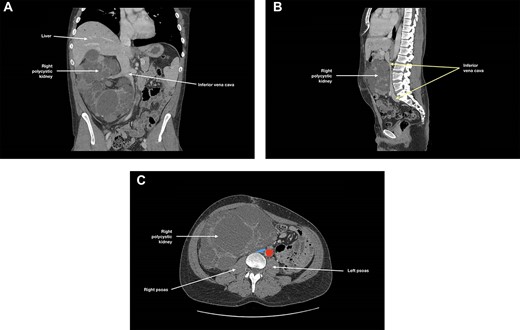
(A) Coronal, (B) sagittal and (C) axial views of the patient’s CT angiogram at 3 months post-left nephrectomy, showing persistent inferior vena cava compression by the right polycystic kidney, with near-complete effacement; in (c), the inferior vena cava is depicted in blue and the descending aorta is depicted in red.
Seven months following right nephrectomy, the patient received renal transplantation from a live paternally related donor. He was discharged 3 days post-transplantation with no immediate complications, good urine output and immediate graft function with stable creatinine [13].
Seventeen days post-transplantation, the patient presented with left lower limb edema. Doppler US identified another episode of DVT extending from left proximal thigh femoral vein to tibioperoneal trunk vein, superficial venous thrombosis in the right proximal thigh to knee region and a 50% stenosis of his right upper arm arteriovenous fistula, requiring fistuloplasty. He was recommenced on lifelong warfarin.
DISCUSSION
This report is significant in presenting a case of (i) recurrent mass effect and compression of major vessels as complications of renal cysts and (ii) recurrent TEs in an ADPKD patient, both in the presence and absence of major vessel compression and despite appropriate anticoagulation.
While enlarged kidneys are common in ADPKD, reports of renal cysts compressing on major vessels are rare, with the authors being only aware of five such cases [6–10]. This is consistent with our single-center experience, where most pretransplant nephrectomies were indicated for spatial considerations of allograft placement, which is in line with practices elsewhere and expert recommendations [14]. However, a recent radiological study identified up to 21% ADPKD patients with extrinsic IVC compression by renal cysts, although any physiologic implications remain to be determined [11].
Virchow’s triad determines that endothelial injury, venous stasis and hypercoagulability are the three main factors predisposing to thromboses. Previous case reports had attributed TEs to hemostasis secondary to major vessel compression [6–10]. In this case, there was worsening of existing DVT and new thromboses despite nephrectomies (which removed extrinsic vessel compression) and anticoagulation. Right polycystic kidney compression of the IVC may have slowed distal venous flow but does not account for subsequent thromboses.
A predilection to TEs in ADPKD patients is supported by previously reported pulmonary embolism in the absence of major vessel compression [15] as well as statistically significant increased risk of TEs among transplanted ADPKD patients [12]. Our single-center experience over 25 years also identified significantly more ADPKD patients who developed thrombotic events compared to other patient groups (manuscript in preparation).
Further characterization of ADPKD patients is required to stratify their risk of mass effect and TEs. This case report raises the question of whether ADPKD patients should progress to lifelong anticoagulation following the first episode of TE and whether prophylactic removal of large polycystic kidneys should be considered, especially given potentially catastrophic outcomes while clinically asymptomatic [6]. Ongoing episodes of TEs in our patient following nephrectomy and appropriate anticoagulation add to the possible association of ADPKD and TEs. This possible association and its underlying pathogenesis warrant further investigation.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



