-
PDF
- Split View
-
Views
-
Cite
Cite
Maher Al Hussan, Zhen Yang, Xinhua Dong, Hongwei Yang, Nanpeng Li, Shishi Qiao, A laparoscopic pancreaticoduodenectomy for pancreatic adenocarcinoma in a patient with situs inversus totalis, Journal of Surgical Case Reports, Volume 2021, Issue 7, July 2021, rjab316, https://doi.org/10.1093/jscr/rjab316
Close - Share Icon Share
Abstract
Situs inversus totalis (SIT) is a congenital disorder in which the thoracic and abdominal viscera organs are mirrored from their normal anatomical position. Thus, the presence of any cancerous mass in one of the visceral organs of patients with SIT represents a great challenge due to the anatomical variation. We report a 52-year-old male with SIT who presented with obstructive jaundice and pancreatic-head mass. After preoperative examinations, it was decided to perform a laparoscopic pancreaticoduodenectomy. In this case, we aim to demonstrate the diagnosis and management of pancreatic cancer in an SIT patient, in addition to presenting the advantages and difficulties of laparoscopic surgery in this case.
INTRODUCTION
Situs inversus totalis (SIT) occurs as a result of congenital chromosomal changes and appears as a complete inverted image of the thoracic and abdominal viscera [1]. The estimated incidence ranges ~1 in 10 000 live births [2], pancreatic adenocarcinoma is one of the deadliest solid malignancies. A large proportion of patients are diagnosed with locally advanced disease or metastatic at the time of presentation, and this unfortunately severely limits the number of patients who can undergo surgical resection [3]. According to The Global cancer statistics (GLOBOCAN) estimates, in 2020 there are 495 773 cases of pancreatic cancer were diagnosed, equivalent to 2.6% of all cancers, and the death rate due to pancreatic cancer in 2020 is estimated at 466 003 cases, equivalent to 4.7% of the death rate from cancer as all [4]. No direct association between SIT and pancreatic cancer has been proven but there have been few reported cases of SIT with pancreatic cancers worldwide [5]. We report an SIT patient with T3N0M0. pancreatic carcinoma. A laparoscopic pancreaticoduodenectomy (LPD) was performed.
| Test . | Result . | Reference range . |
|---|---|---|
| White blood cells | 5.06 | 3500–9500/μl |
| Neutrophils percent | 68.1% | 40–75% |
| Hemoglobin | 137.0 | 130–175 g/dl |
| Hematocrit | 0.398 ↓ | 0.4–0.5 |
| Platelets | 212 | 150–450 × 103/μl |
| Urea | 32 | 10–50 mg/dl |
| Creatinine | 0.72 | 0.2–1.15 mg/dl |
| ALT | 119↑ | 0–40 IU/l |
| AST | 68 ↑ | 0–40 IU/l |
| GGT | 687↑ | 0–58 IU/l |
| ALP | 214↑ | 40–130 IU/l |
| TBIL | 95.6↑ | 0–25 μmol /l |
| BILD2 | 77.4↑ | 0–10 μmol /l |
| IBIL | 18.↑ | 0–14 μmol /l |
| CA19_9 | 270↑ | 0.01–37 |
| CEA | 73.2↑ | 0–5 |
| AFP | 2.48 | 0–10 |
| CA72-4 | 55.3↑ | 0–6.9 |
| Test . | Result . | Reference range . |
|---|---|---|
| White blood cells | 5.06 | 3500–9500/μl |
| Neutrophils percent | 68.1% | 40–75% |
| Hemoglobin | 137.0 | 130–175 g/dl |
| Hematocrit | 0.398 ↓ | 0.4–0.5 |
| Platelets | 212 | 150–450 × 103/μl |
| Urea | 32 | 10–50 mg/dl |
| Creatinine | 0.72 | 0.2–1.15 mg/dl |
| ALT | 119↑ | 0–40 IU/l |
| AST | 68 ↑ | 0–40 IU/l |
| GGT | 687↑ | 0–58 IU/l |
| ALP | 214↑ | 40–130 IU/l |
| TBIL | 95.6↑ | 0–25 μmol /l |
| BILD2 | 77.4↑ | 0–10 μmol /l |
| IBIL | 18.↑ | 0–14 μmol /l |
| CA19_9 | 270↑ | 0.01–37 |
| CEA | 73.2↑ | 0–5 |
| AFP | 2.48 | 0–10 |
| CA72-4 | 55.3↑ | 0–6.9 |
| Test . | Result . | Reference range . |
|---|---|---|
| White blood cells | 5.06 | 3500–9500/μl |
| Neutrophils percent | 68.1% | 40–75% |
| Hemoglobin | 137.0 | 130–175 g/dl |
| Hematocrit | 0.398 ↓ | 0.4–0.5 |
| Platelets | 212 | 150–450 × 103/μl |
| Urea | 32 | 10–50 mg/dl |
| Creatinine | 0.72 | 0.2–1.15 mg/dl |
| ALT | 119↑ | 0–40 IU/l |
| AST | 68 ↑ | 0–40 IU/l |
| GGT | 687↑ | 0–58 IU/l |
| ALP | 214↑ | 40–130 IU/l |
| TBIL | 95.6↑ | 0–25 μmol /l |
| BILD2 | 77.4↑ | 0–10 μmol /l |
| IBIL | 18.↑ | 0–14 μmol /l |
| CA19_9 | 270↑ | 0.01–37 |
| CEA | 73.2↑ | 0–5 |
| AFP | 2.48 | 0–10 |
| CA72-4 | 55.3↑ | 0–6.9 |
| Test . | Result . | Reference range . |
|---|---|---|
| White blood cells | 5.06 | 3500–9500/μl |
| Neutrophils percent | 68.1% | 40–75% |
| Hemoglobin | 137.0 | 130–175 g/dl |
| Hematocrit | 0.398 ↓ | 0.4–0.5 |
| Platelets | 212 | 150–450 × 103/μl |
| Urea | 32 | 10–50 mg/dl |
| Creatinine | 0.72 | 0.2–1.15 mg/dl |
| ALT | 119↑ | 0–40 IU/l |
| AST | 68 ↑ | 0–40 IU/l |
| GGT | 687↑ | 0–58 IU/l |
| ALP | 214↑ | 40–130 IU/l |
| TBIL | 95.6↑ | 0–25 μmol /l |
| BILD2 | 77.4↑ | 0–10 μmol /l |
| IBIL | 18.↑ | 0–14 μmol /l |
| CA19_9 | 270↑ | 0.01–37 |
| CEA | 73.2↑ | 0–5 |
| AFP | 2.48 | 0–10 |
| CA72-4 | 55.3↑ | 0–6.9 |
CASE PRESENTATION
A 52-year-old male had no obvious inducement of epigastric pain for >3 months presenting persistent stabbing pain, accompanied by back pressure, fatigue, loss of appetite and hiccup. He went to a local hospital for treatment because of aggravated abdominal pain. Gastroscopy showed: (i) gastric polyps and (ii) chronic atrophic gastritis. The symptoms were not relieved after oral medication. Two weeks later he had jaundice with clay-like stool, so he went to the local hospital and underwent a color Doppler ultrasound examination. The results showed that: (i) visceral inversion; (ii) solid space-occupying at the head of the pancreas; (iii) dilated inner diameter of the intrahepatic and extrahepatic bile ducts and main pancreatic duct; (iv) fatty liver and (v) enlarged gallbladder volume and wall rough. The patient was admitted to our hospital complaining of ‘epigastric pain >3 months ago, jaundice with clay-like stool for 1 week’. The patient had no significant medical, surgical or drug history. Physical examinations were unremarkable except for a weight loss of ~20 kg since the onset of the disease. Blood and laboratory tests were carried out, and they showed elevated values of liver enzymes, with a clear elevation in the values of Enzyme gamma-glutamyl transferase (GGT) and alkaline phosphatase (ALP), as well as tumor markers CA19-9, CEA and CA72-4, which are indicative of the presence of an obstructive lesion (Table 1). After preoperative examinations, he was diagnosed with pancreatic cancer. His CT scan (Fig. 1) showed: (i) mirror dextrocardia; total visceral inversion; (ii) pancreatic space-occupying lesions; (iii) intrahepatic and extrahepatic bile duct dilation; (iv) intrahepatic calcification and (v) multiple small cysts in the left kidney, and the MRI scan has confirmed the previous results (Fig. 2). A perioperative plan has been made for a LPD. The patient was put under general anesthesia in a supine split–leg position. The laparoscopic instruments were connected (Fig. 3). Diagnostic laparoscopy confirmed a mirror transportation of organs hence confirming SIT, considering the preoperative assessments and intraoperative exploration, a LPD, and intestinal adhesiolysis was performed. Major vessels along with thoracic and abdominal organs were transposed as mirror images of the normal anatomy, a 17-cm paramedian incision in the upper left abdomen was made to accomplish the resection of the organs which includes the distal stomach—the duodenum, the upper segment of the jejunum, the common bile duct and the gallbladder. According to conventional loop reconstruction, the reconstruction of the alimentary tract was performed. Three drainage tubes were placed at the sites of the anastomoses. Although there were anatomical variations, the operation went smoothly. The blood pressure of the patient was stable and the anesthesia effect was satisfactory within 150-ml intraoperative blood loss. The postoperative specimens were sent to the pathological lab. Postoperative fluoroscopy was normal (Fig. 4). The patient had a total hospital stay of 26 days; 15 days postoperative hospital stay, and was discharged without any complications.
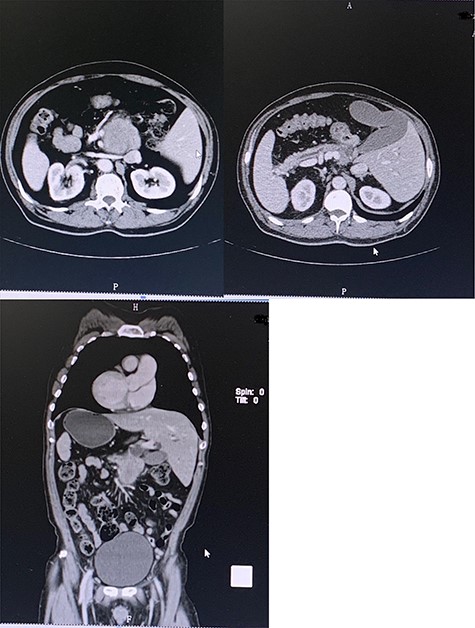
Preoperative contrast-enhanced CT confirming SIT with space-occupying at the head of pancreas and duodenum.
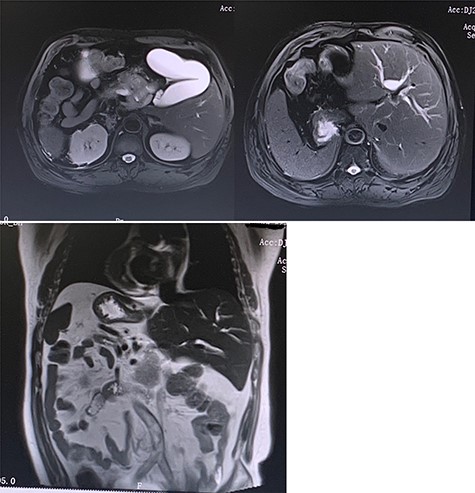
Preoperative MRI (T2 phase) confirming the SIT and showing the intrahepatic bile ducts dilation and the enlarged gallbladder with a space-occupying at the head of the pancreas.
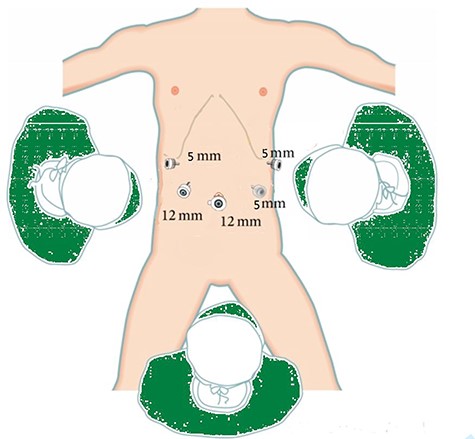
Showing the position of the chief surgeon (right side of the patient) with his assistants, the laparoscope hole below the umbilicus and four trocars holes were placed at the right and left abdomen.
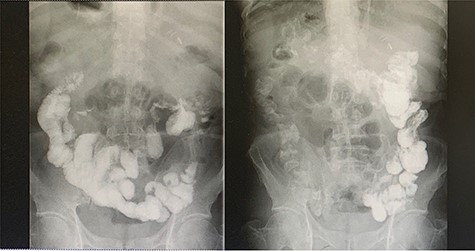
Left-image is postoperative fluoroscopy confirming smooth passage of contrast in the remnant stomach and the gastrojejunostomy with no stenosis or flaccidity; Right-image confirming smooth passage of contrast in the choledochojejunostomy and pancreaticojejunostomy.
Postoperative pathological report (Fig 5) indicated the following:
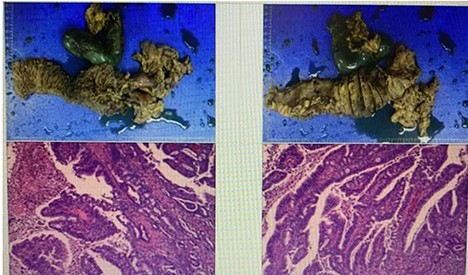
Tumor site: pancreatic-head.
Pathological stage: T3N0M0.
Tumor size: 7.3 × 6.8 × 3 cm.
Histological type: ductal adenocarcinoma.
Histological grade: grade IIA.
Surgical margin invasion: there is a cancer involvement found into the duodenal wall, no cancer involvement found in the gastric cutting edge, common bile duct stump.
Lymph node involvement: no lymph node involvement.
Nerve invasion is present. However, no vascular invasion was seen.
DISCUSSION
SIT is a rare congenital disorder that is represented by total mirror-image transportation of the thoracic and abdominal viscera [1, 2]. The anomalies manifestations associated with SIT generally are uncommon, perhaps the most prominent of which are polysplenia and the interruption of the inferior vena cava (IVC; [6]), however, there is no direct relationship between SIT and the presence of carcinomas. In our case, it was found that there was a mass on the head of the pancreas and after thorough examinations, the patient was diagnosed with pancreatic ductal adenocarcinoma. It is one of the most lethal cancers for several reasons, including the delayed diagnosis due to the delay in the appearance of symptoms and the intersection of symptoms with many other diseases. Furthermore, the sensitive anatomical site makes the rate of invasion to neighboring organs greater [1, 3]. Surgical treatment is considered the only curative treatment for tumors of the head of the pancreas. It is represented by a pancreaticoduodenectomy (Whipple procedure). LPD is more advantageous than traditional surgery as it gives a wider field of vision and is minimally invasive but it is difficult to perform and requires a lot of precision and dexterity [7]. In our case, the presence of SIT poses a great challenge due to the necessary changes in the technique used [8] as well as anatomical variations that were not expected before surgery [9] specifically, the different sites and pathways of blood vessels that represent a challenge to the surgeon require great accuracy during the operation [10]. Despite these difficulties, many surgical cases associated with SIT have been performed successfully via laparoscopic interventions [11]. In the end we conclude that LPD in SIT patients is feasible, taking into account the necessary technical changes as well as the sufficient expertise, accuracy and dexterity required to perform this procedure.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING
Professor Zhen Yang was supported by first affiliated hospital of Zhengzhou University, Zhengzhou, Henan, China.
ETHICAL APPROVAL
The study is exempted from ethical approval.
CONSENT STATEMENT
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.



