-
PDF
- Split View
-
Views
-
Cite
Cite
Jacqueline B Baikovitz, Lindsay Thornton, Monica T Garcia-Buitrago, Alan S Livingstone, Matthew T Studenski, Lorraine Portelance, Pathologic validation of an yttrium-90 trans-arterial radioembolization dosimetry: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 4, April 2021, rjab078, https://doi.org/10.1093/jscr/rjab078
Close - Share Icon Share
Abstract
Yttrium-90 (Y-90) trans-arterial radioembolization (TARE) is used in the management of unresectable hepatocellular carcinoma (HCC). During the last 5 years, dosimetry software has been developed to allow for a more rigorous approach of dose prescription in Y-90 TARE. We present here a case study of a 77-year-old woman diagnosed with HCC, who underwent a Y-90 TARE as a bridge procedure to liver resection. This clinical scenario represents a unique opportunity to illustrate the predictive value of dosimetric findings correlating dosimetry with pathological findings. In this case, Y-90 TARE dosimetry was predictive of treatment response in which the tumor received a mean dose of 156 Gy and demonstrated a complete pathologic response.
INTRODUCTION
Yttrium-90 (Y-90) trans-arterial radioembolization (TARE) has been used in the management of liver primary cancer (hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma) and liver metastasis for many decades [1]. Dose calculation has traditionally been performed based on empirical calculation models. During the last 5 years, dosimetry software has been developed to allow for a more rigorous approach of dose prescription. This software takes into account the precise volumetric measure of the gross tumor volume (GTV), the perfused treatment volume, the tumor-to-normal tissue (TN/T) ratio and the liver lung shunt [2]. The treatment planning software also allows for early assessment of treatment results by performing post-treatment dosimetry using a liver positron emission tomography (PET) or single-photon-emission computed tomography (SPECT)/computed tomography (CT) Bremsstrahlung SPECT. Two products have been approved by the United States Food and Drug Administration (FDA) in the last 2 years (SurePlan, MIM System Cleveland, OH, and Planet, Dosisoft System, Cachan, France). We present here a case study of a patient diagnosed with HCC who underwent a Y-90 TARE as a bridge procedure to liver resection. This clinical scenario represents a unique opportunity to illustrate the predictive value of dosimetric findings correlating dosimetry with pathological findings.
CASE
A 77-year-old woman was diagnosed with HCC in January 2019. Her past medical history was significant for coronary artery disease, including coronary artery bypass grafting in 2008 and coronary stent placement in 2009, hypertension, rheumatoid arthritis, chronic kidney disease Stage III and invasive ductal carcinoma of the left breast cancer (T1bN0N0, ER/PR+, HER2-) treated with lumpectomy adjuvant radiation in 2017 and for which she has been taking letrozole daily. Past medical history was also notable for hepatitis C virus (HCV) diagnosed in November 2015 following workup for elevated liver function tests. The patient had a remote history of blood transfusion in 1997 at the time of a partial colectomy for gastrointestinal malrotation and bowel ischemia and the assumption was that she then contracted hepatitis. Her HCV was successfully eradicated with ledipasvir/sofosbuvir in May 2016.
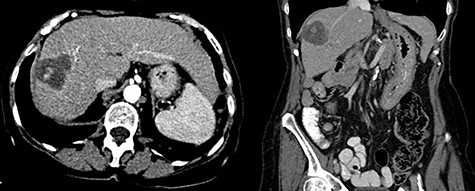
In 2018, the patient had a low dose chest CT for lung cancer screening, given her prior smoking history. The chest CT demonstrated a mass along the right hepatic dome. Triple-phase contrast-enhanced (ce) CT of the abdomen subsequently revealed a large multi-lobulated low-attenuation mass in the right hepatic lobe, predominantly involving Segments V and VIII and, to a lesser extent, Segments VI and VII, measuring 8.8 × 8.3 × 8.2 cm, and associated with a satellite lesion in Segment IVA, measuring 2.2 cm (Fig. 1). A PET CT was ordered and was negative for extrahepatic neoplastic disease.
Laboratory data at diagnosis included negative hepatitis C ribonucleic acid (RNA) quantitative polymerase chain reaction (PCR) study, normal liver function tests and normal prothrombin time (PT)/INR. Alpha fetoprotein (AFP) was elevated at 119 ng/ml (normal AFP < 8.3 ng/ml) and carbohydrate antigen (CA) 19-9 was also elevated at 125.5 U/ml (normal CA: 19-9: 0-35 U/ml). The patient subsequently underwent a CT-guided liver biopsy that showed histological features consistent with HCC (Fig. 2).
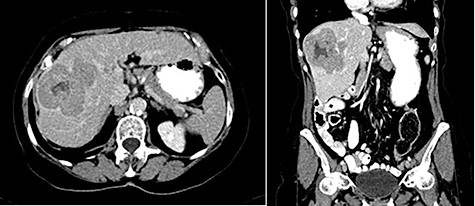
Abdominal ceCT demonstrated an arterially enhancing mass with early washout characteristic of HCC; venous phase axial and coronal images are featured.
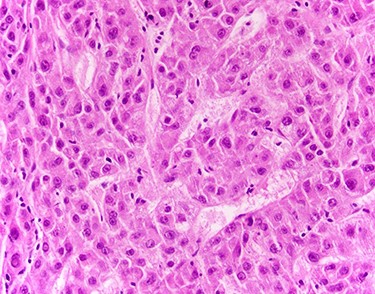
Liver biopsy showed neoplastic cells with cytoplasmic amphophilia, increased nuclear/cytoplasmic ratio and an abnormal trabecular architecture, diagnostic of HCC.
The patient had an Eastern Cooperative Oncology Group (ECOG) Performance Status score of 1, having symptomatic rheumatoid arthritis, Child-Pugh A (score of 5) and Barcelona Clinic Liver Cancer (BCLC) Stage B and TNM Stage IIIA based on imaging.
The consensus from the multidisciplinary liver tumor board was to proceed with Y-90 TARE to achieve tumor shrinkage and to induce left liver lobe hypertrophy in preparation for surgical resection. In February 2019, the patient underwent a Y-90 TARE, and a dose of 2.71 GBq (73.4 mCi) of glass microspheres (TheraSpheres, Boston Medical) was injected into the right hepatic artery (Fig. 3). The delivery of the dose was verified using Bremsstrahlung liver SPECT imaging post-injection (Fig. 4). Based on this activity distribution, the dose was calculated on a voxel-by-voxel basis using Sure Plan software (MIM, Cleveland Ohio). The local deposition method (LDM) was used, and the dose was scaled by the amount of activity injected.
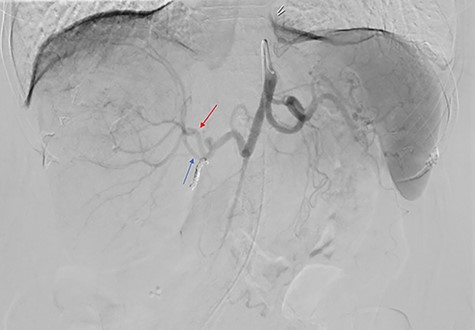
Celiac angiogram demonstrated tumor supply from both the anterior (red arrow) and posterior (blue arrow) divisions of the right hepatic artery; the gastroduodenal artery was coiled for embolic protection.
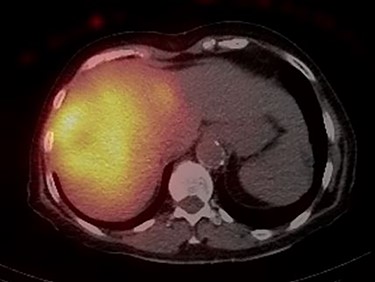
Post-Y-90 Bremsstrahlung SPECT/CT demonstrates uptake predominantly within the main right liver lobe lesion (GTV) with minor uptake into the Segment IVA lesion.
Three months post-radioembolization, ceCT abdomen and pelvis showed that the large right hepatic lobe mass was almost entirely necrotic with central hemorrhage and was decreased in size, measuring 4.2 cm. The right hepatic artery provided minor supply to the Segment IV lesion, and therefore this lesion had also decreased in size from 1.8 to 1 cm (Fig. 5). There were no additional hepatic lesions demonstrated. The AFP level dropped from 119 pre-treatment to 8.4 ng/ml (normal AFP < 8.3 ng/ml).
Three month follow-up ceCT demonstrates necrosis and involution of the lesion as well as spontaneously hyperdense material centrally consistent with hemorrhage.
An extended right hepatectomy was performed 4 months post-Y-90 treatment. The main lesion in the right liver lobe was found to be completely necrotic and showed extensive fibrosis, while viable tumor tissue was seen only in the small Segment IVA nodule. This residual lesion measured 7 mm. All resection margins were negative for carcinoma. There was extensive fibrosis, with ductular reaction surrounding the necrotic nodule (Fig. 6).
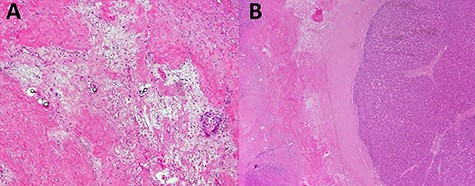
Sections from partial hepatectomy; (A) tumor area of extensive hyalinized fibrosis and glass microspheres; (B) small focus of residual HCC adjacent to fibrosis.
The postoperative course was uneventful notably without hepatic encephalopathy, gastrointestinal bleeding or ascites formation. The liver function tests remained within normal limits. The AFP further dropped to 4.8 and 4.4 ng/ml, at 1 month and 6 months respectively, following surgery. Six months postoperatively, abdominal ceCT scan did not show residual tumor (Fig. 7).
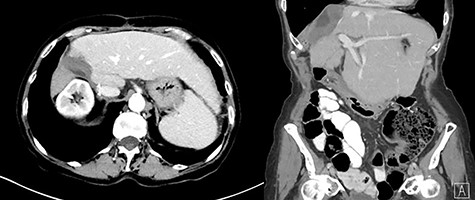
Six-month postoperative ceCT showing no residual tumor; post-surgical changes are seen along the cut edge of the liver status post right hepatectomy.
Post-treatment dosimetry done using the Bremsstrahlung liver SPECT image and the Sure Plan software demonstrated that the mean dose delivered to the main right liver lobe tumor (GTV) was 156 Gy. Ninety-five percent of the GTV received 108 Gy with a max dose of 213 Gy. The mean dose to the Segment IVA lesion was only 62 Gy, with 95% of the lesion receiving 36 Gy (Figs 8 and 9).
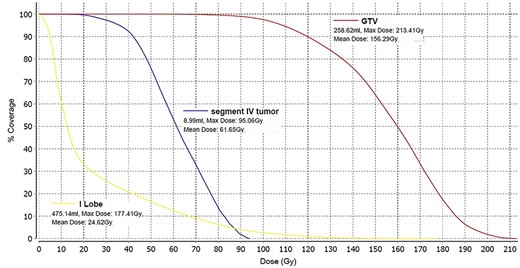
Dose–volume histogram of the main lesion (GTV) localized in the right liver lobe (red), the dose to the Segment IVA lesion (blue) and the dose to the left liver lobe (yellow).
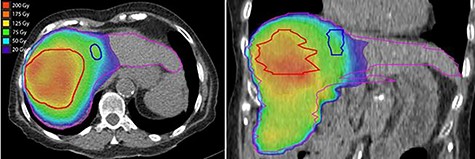
Axial and coronal views of the Y-90 dose distribution; the majority of the uptake is within the GTV with minor uptake in the Segment IVA lesion.
DISCUSSION
In this case report, we demonstrate that the post-Y-90 TARE dosimetry was predictive of treatment response. The main tumor which received a mean dose of 156 Gy showed a complete pathologic response, while the satellite nodule that was treated to a mean dose of 62 Gy showed only a partial response.
Since this was a procedure performed in preparation for a right lobectomy, a mean dose to the right liver lobe of 74 Gy was deemed acceptable. The left liver lobe was almost totally spared from any radiation with a mean dose of 25 Gy and 95% of the lobe receiving 3.5 Gy. These findings are in agreement with other published data indicating that delivering a dose higher that 100 Gy during Y-90 TARE leads to good tumor control [2]. The benefit of using Y-90 TARE to deliver an ablative radiation dose is that this treatment technique completely spares the surrounding extra hepatic organs from radiation. The use of dosimetry software brings important data for outcome analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflicts of interest. ICMJE Form for Disclosure of Potential Conflicts of Interest is included for each author.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
AUTHORS’ CONTRIBUTIONS
J.B.B. reviewed the patient’s case and described the patient’s clinical presentation and treatment management and outcomes. L.T. provided and described the hepatic arterial mapping and CT and PET imaging. M.T.G.-B. commented on the liver pathology. A.S.L. wrote the details of the right hepatic lobectomy and surgical outcomes. M.T.S. performed the dosimetry calculations and conducted the dosimetric evaluation. L.P. detailed the Y-90 TARE procedure and wrote the discussion on Y-90 dosimetry. All authors read, revised and approved the final manuscript.



