-
PDF
- Split View
-
Views
-
Cite
Cite
Jared M Wohlgemut, Martin Hennessy, Keith Hussey, Thrombolysis for acute graft occlusion during elective endovascular aortic aneurysm repair, Journal of Surgical Case Reports, Volume 2020, Issue 3, March 2020, rjaa056, https://doi.org/10.1093/jscr/rjaa056
Close - Share Icon Share
Abstract
A 65-year-old man developed acute arterial thrombosis with stent graft occlusion, during elective endovascular aneurysm repair, with bilateral acute lower limb ischaemia. We describe successful endovascular and pharmacological management using a combination of mechanical disruption of the thrombus (using the access sheaths) followed by intra-arterial thrombolysis (Actilyse) infusion. Within 4-h the endograft had completely re-canalized. The patient made an uncomplicated recovery and was discharged on the second post-operative day.
INTRODUCTION
Graft thrombosis a rare complication of endovascular aneurysm repair (EVAR) associated with significant morbidity and mortality [1]. Lower extremity ischaemia can result in disabling intermittent claudication or acute limb threatening ischaemia. Proximal propagation of thrombus may compromise the visceral segment [1]. There are endovascular and open therapeutic options, which are dictated by both the clinical presentation and chronicity of the problem. We describe a case of acute graft thrombosis evident at completion angiography and a novel endovascular solution, which was performed to good effect.
CASE REPORT
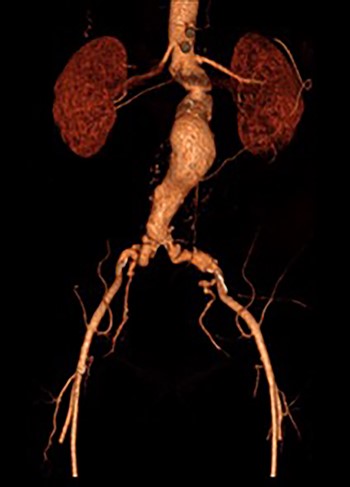
A 65-year-old man was admitted electively with a 55-mm infrarenal abdominal aortic aneurysm (AAA). Computed tomography (CT) angiography had demonstrated aneurysm morphology that was suitable for EVAR, although there were some adverse features with the neck (16 mm length, but conical and angulated) (Fig. 1). His past medical history was significant and included type 2 diabetes mellitus, obesity (body mass index of 32.2 kg/m2), hypercholesterolaemia, ischaemic heart disease (with coronary artery bypass graft) and associated moderate left ventricular systolic dysfunction, chronic obstructive pulmonary disease, arthritis and poor mobility requiring a wheelchair. Following discussion in the local multidisciplinary team meeting a decision was made to offer percutaneous EVAR under local anaesthesia.
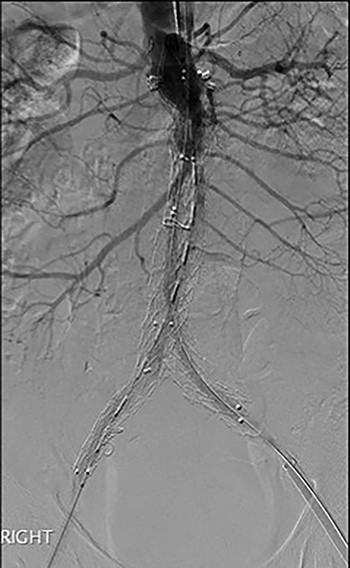
Post-EVAR angiogram showing acute thrombosis of the right limb and subtotal thrombosis of the left limb and trunk.
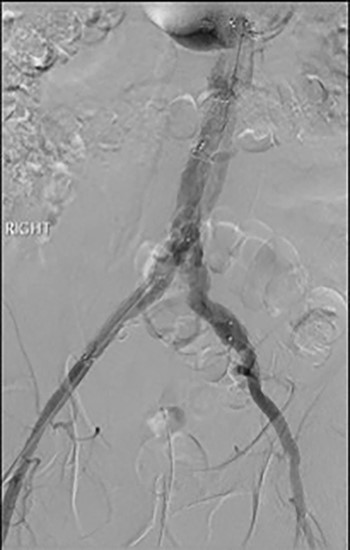
Post-lysis CT scanning showing fully re-canalized aorto-iliac system.
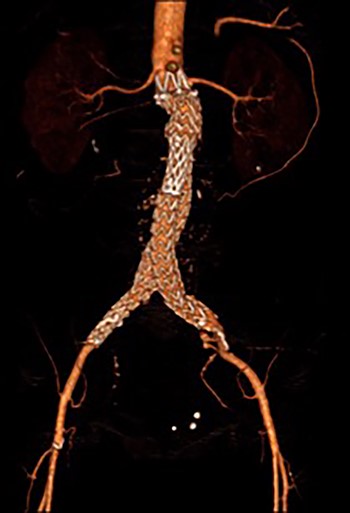
3D reconstruction CT scan, at 6-week interval from index procedure.
The procedure was performed under local anaesthetic (10 mls 1% Lidocaine and 10 mls 0.25% Chirocaine) to each groin. Percutaneous access was established under ultrasound guidance and each access site was preclosed, with two Proglides (Abbott, Santa Clara, CA, USA), followed by bilateral 7Fr access. When access had been established 3000 units of intravenous unfractionated heparin was administered by the anaesthetic team. On the right side, a decision had been made to extend the endograft to the right external iliac artery. The right internal iliac artery was cannulated and coil embolisation performed using three 8 × 50 mm Cook (Bloomington, IN, USA) MReye embolisation coils. The left sheath was up-sized to 16 Fr Sentrant (Medtronic, Minneapolis, MN, USA) sheath; the main body of the device was introduced from the right femoral access. The primary deployment was uncomplicated, although cannulation of the gate of the endograft proved to be challenging. During this time, the patient became unsettled reporting back and leg pain. A decision was made to convert to general anaesthesia at this stage. Following this, the gate cannulated and the remainder of the endograft deployment was unremarkable.
A Reliant (Medtronic) molding balloon was used and the aortic bifurcation managed with ‘kissing’ 12 × 40 mm angioplasty balloons as there was atherosclerotic disease at this level. Given the patient’s relative youth and the unfavourable neck characteristics a decision was made to use Endoanchors (Medtronic.) Six were placed in accordance with the manufacturers’ ‘Instructions for Use’.
An angiogram at this stage demonstrated acute thrombosis of the right limb, and subtotal thrombosis of the left limb (Fig. 2). A further bolus of 2000 units Heparin was given intravenously. Thrombectomy was performed by advancing the 16Fr Sentrant sheaths into the limbs of the endograft followed by retraction under negative pressure. This manoeuvre restored antegrade arterial flow on both sides although there was a large volume of acute thrombus within the limbs of the endograft. A decision was made to perform intra-arterial thrombolysis. The 16Fr Sentrant sheath was removed from the left common femoral artery and haemostasis secured with the Proglides. The right-sided 16Fr sheath was downsized to 8 Fr sheath (the Proglide sutures were tightened around this sheath, achieving haemostasis around the smaller sheath). A multi-side hole straight angiographic catheter was placed in the body of the endograft for the thrombolysis. A bolus of 5 mg Actilyse (Boehringer Ingelheim, Ingelheim am Rhein, Germany) was given through this followed by a 1 mg/h infusion. An IV heparin infusion was started at 1000 units/h through the side arm of the 8 Fr sheath.
The patient was transferred to the high-dependency unit for observation during the thrombolysis. After 4-h, the patient was taken back to Interventional Radiology department for a check angiogram (Fig. 3). This demonstrated a fully re-canalized aorto-iliac system with no distal thrombotic or embolic complications. The thrombolysis infusion was stopped at this stage. In the post-operative period, anticoagulation has been continued with Apixaban. A CT scan 6 weeks post-procedure has confirmed ongoing patency of the endograft with no visible thrombus (Fig. 4).
DISCUSSION
This was an unexpected complication, which was probably multifactorial in origin. The heparin dose was probably inadequate, the duration of the procedure was longer than anticipated and there was concurrent obstructive atherosclerotic arterial disease.
To our knowledge, this is the first description of on-table management of acute thrombosis during the index procedure. Thrombolysis has been described previously when a patient presented with acute limb ischaemia 3 years after the index procedure [2]. The risks of intra-arterial thrombolysis include intracranial haemorrhage (0.5%), gastro-intestinal haemorrhage, hypersensitivity reactions [3], hypotension, myocardial rupture [4], peripheral embolization [5] and failure of clot disintegration. Relative contra-indications of catheter directed thrombolysis include recent major surgery, and recent arterial or venous puncture of non-compressible vessels [6]. Alternative therapeutic strategies include thrombectomy via bilateral common femoral artery exposure—which negates the benefits of percutaneous approach on morbidity—or open surgery and graft explantation, which is associated with significant perioperative morbidity and mortality (range 9–37%), especially in the non-elective setting [7–8]. In the absence of a contra-indication to catheter directed thrombolysis, this was considered to be the most elegant management strategy, following mechanical restoration of antegrade flow with aspiration thrombectomy using the 16Fr Sentrant sheaths, as this could be achieved percutaneously with re-course to surgical exposure of the common femoral arteries if required (which in the context of obesity would have been associated with the potential for wound infection).
In conclusion, we present a satisfactory outcome for immediate endograft thrombosis with a combination of mechanical/aspiration thrombectomy and intra-arterial catheter direct thrombolysis. This management plan restored the distal perfusion, but importantly maintained all of the benefits of an endovascular strategy in a patient with significant medical co-morbidity.
ACKNOWLEDGEMENTS
The authors have no acknowledgements of persons or institutions.
Conflict of interest statement
None declared.



