-
PDF
- Split View
-
Views
-
Cite
Cite
Nader Abraham, Ayman S Nabawi, The challenging case of a primigravid Bedouin woman with a dormant neck nodule that grew explosively during her pregnancy, Journal of Surgical Case Reports, Volume 2019, Issue 3, March 2019, rjz061, https://doi.org/10.1093/jscr/rjz061
Close - Share Icon Share
Abstract
A Desmoid tumor in the neck is a rare tumor in an even more unusual site. Our patient was an 18-year-old primigravid Bedouin woman from northwest Egypt. She presented with a dormant neck nodule that grew explosively during her first pregnancy and stopped abruptly after delivery. The presentation was confusing at first, as the fixation of the tumor to the underlying tissues implied a malignancy, while a 1-year history of non-metastasis alluded to a benign process. Pre-operative tissue diagnosis revealed an Estrogen receptor-expressing desmoid tumor. Desmoid tumors are indeed locally invasive with no metastatic potential, but they tend to recur and grow during high-estrogen states. This report aims to increase awareness of peripartum Desmoid tumors, as well as discuss the surgical-site, psychological and socioeconomic challenges in the peculiar case of this Bedouin woman, and our recommendations after this experience and literature review.
INTRODUCTION
Desmoid tumors (DTs) are rare, locally infiltrative, fibroblastic proliferative disease, with an estimated incidence of 2–4 cases per million per year, and a total prevalence of 0.03% of all neoplasms [1]. DTs usually arise from abdominal fascial tissue, but in the rare occasions of extra-abdominal occurrence, the neck, shoulder girdle, chest wall and inguinal region are the most common sites [2]. DTs were observed to express estrogen and anti-estrogen receptors, as well as proliferate both in vivo and in vitro in high-estrogen states; which is thought to be the reason behind the higher growth rate in pregnancy and women on OCPs over non-pregnant women and males. DTs were also reported to regress after menopause and with tamoxifen treatment [3]. DTs occur both as isolated and syndromic, i.e. Gardner’s syndrome [4]. Both ways are believed to be the result of the Wnt signaling pathway activation through different mechanisms [5].
CASE REPORT
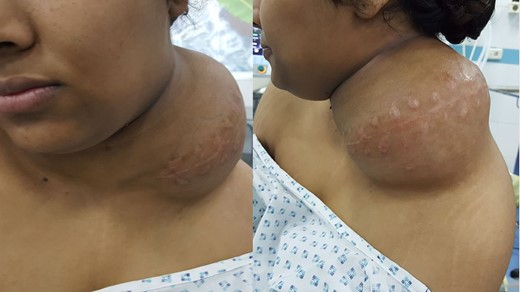
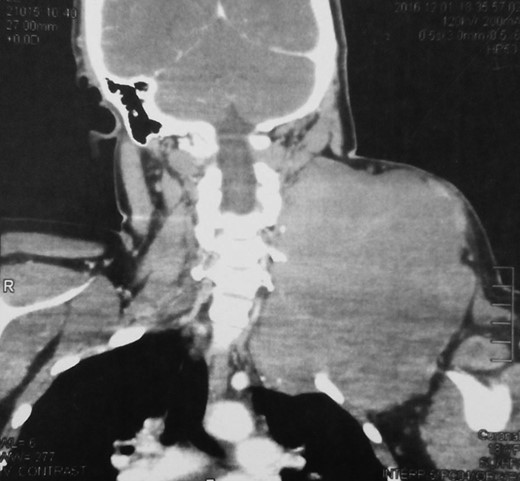
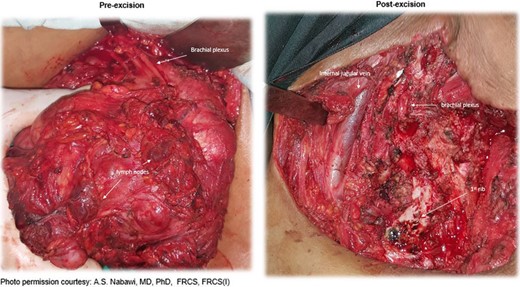
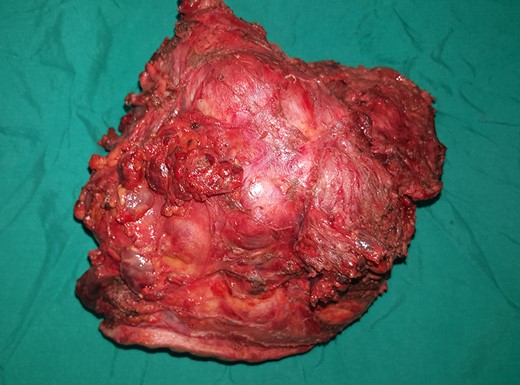
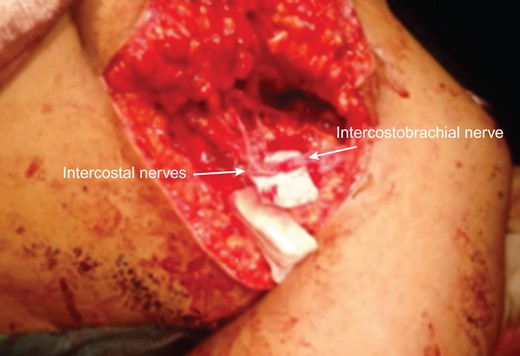
An 18-year-old primigravid Bedouin woman was admitted for resection of a 15 × 10 cm symptomatic, deforming solid mass fixed to her neck and left shoulder girdle (Fig. 1). Past medical history revealed a 1 × 1 cm dormant nodule, felt in her left sternomastoid, which grew explosively during her first pregnancy and stopped abruptly after delivery, with no history of major antecedent trauma. Physical examination showed limited abduction of her left shoulder, left arm weakness, forearm numbness, numbness of the three medial fingers and left wrist drop (Fig. 2). The tumor extended deeply medially into the neck causing right-sided deviation of the thyroid and trachea, with associated dysphagia to solids, but no change in voice. CT scanning revealed a mass attenuation inseparable from the paravertebral muscles extending into the left apical pleura (Fig. 3). MRI discerned that the tumor was not pressing on the brachial plexus, but rather infiltrating it. Pre-operative core-needle biopsy demonstrated a benign spindle cell neoplasm, consistent with a desmoid tumor. Immunohistochemical staining for estrogen receptors demonstrated a moderate positive reaction of most of the neoplastic cells. The patient underwent en bloc resection by dissection from the overlying skin, left internal jugular vein, left sternomastoid and trapezius muscles, left first rib, left apical pleura, and the left paravertebral muscles and cervical nerve roots. The tumor-encased brachial plexus was unsalvageable (Fig. 4). The incision closure included revision of the scar of a recently failed resection and insertion of a closed suction drain. Pathological evaluation of the excised tumor confirmed a 15 × 10 × 9 cm, non-encapsulated tumor with several enlarged, reactive-type cervical lymph nodes (Fig. 5). The patient’s postoperative course was notable for paralysis of the left upper limb, and a Chyle leak (trauma to the thoracic duct) through the drain, which stopped spontaneously 10 days after the operation (Fig. 6). A month after the resection, the patient underwent nerve transfer to salvage sensation to her left hand and the flexion–extension function around the elbow. Three intercostal nerves, along with the intercostobrachial nerve, were transposed and attached to the median nerve of the affected arm (Fig. 7).
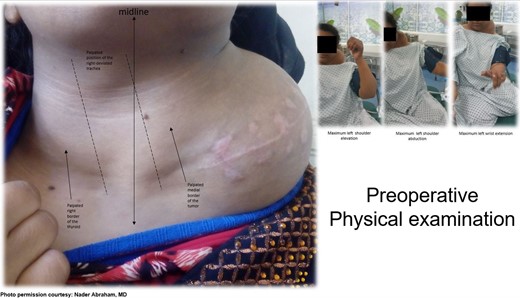
Pre-operative physical examination of the neck and left shoulder.

DISCUSSION
Two months after the nerve transfer, the patient reported a gradual perception of tingling in her previously numb left palm, a sign of ongoing successful reinnervation. Unfortunately, we witnessed not the full neuromuscular recovery of the patient. Given the syndromic DTs and FAP association [6], we recommended a colonoscopy to rule out colonic lesions. Young age, coupled with fear, Illiteracy and strict upbringing proved insurmountable barriers in explaining and understanding such association and undergoing the colonoscopy. Ultimately, we lost her to follow up. It was even difficult to obtain any relevant familial history of colonic lesions in her 10 older siblings, due to either true unawareness or fear of dishonoring and disreputing the health of her family. With an estrogen-and-trauma-sensitized locally infiltrative tumor, paralyzed left arm and a female first-born, she faced the dire risk of legal polygamy or even divorce if she fails to produce the highly sought-after male child in her marginalized Bedouin society. Therefore, early in the management plan, we had to consider the potential profound social repercussions of her condition and reviewed the literature to assess the prospect of a recurrence-free conception. In 2014, an international, multi-institutional Analysis of Recurrence and Obstetric Risk reported a progression or recurrence in 42% of women with a history of DTs with pregnancy [7]. Though a DT is not an automatic contraindication to a subsequent pregnancy, with such surgical trauma, and with coin toss-like odds, we erred on the side of caution and tried to minimize the levels of estrogen that any remaining tumor cells would encounter. We shifted her away from her current OCPs to a copper IUD for long-term contraception and prescribed Tamoxifen [8]. If the patient would ever return with a recurrence, our management plan would be non-surgical. DTs are radiosensitive, and in a patient where surgical morbidity is high, local irradiation would be an acceptable definitive therapy for local recurrence control. However, it is not free from its late risks, especially in this a young age, and regression could take several years [9]. Sorafenib is another even slower non-surgical option. After a phase 3 trial, it recently showed to offer significantly prolonged progression-free survival, and more durable responses [10].
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
Constantinidou A, Scurr M, Judson I, Litchman C.



