-
PDF
- Split View
-
Views
-
Cite
Cite
Nitin Sharma, Wai Yip Chau, Lisa Dobruskin, An unusual case of perisplenic small bowel volvulus after laparoscopic Roux En Y gastric bypass, Journal of Surgical Case Reports, Volume 2019, Issue 2, February 2019, rjz042, https://doi.org/10.1093/jscr/rjz042
Close - Share Icon Share
Abstract
Internal hernias (IH) are one of the dreadful complications of laparoscopic Roux En Y gastric bypass (LGBP). Commonly reported internal hernias (IH) following Roux En Y gastric bypass (LGBP) in the literature are meso-colic, meso jejunal and Peterson’s space hernias. These patients may not have any definitive symptoms. Findings are often missed on radiological studies and a high index of clinical suspicion is often necessary. If in doubt, a timely diagnostic laparoscopy is critical to decrease morbidity and mortality in these patients. We present a very unusual case of peri-splenic small bowel herniation with volvulus following LGBP with indeterminate radiological findings. Our case emphasizes that early laparoscopy is both diagnostic and therapeutic for desirable clinical outcomes.
INTRODUCTION
Laparoscopic Roux-en-Y gastric bypass (LGBP) is the second most performed surgical procedure for morbid obesity in USA. It is effective both in terms of effective weight loss and improvement in comorbidities. Laparoscopic approach offers many advantages in terms of fewer wound complications, decreased length of hospital stay, and decreased postoperative pain. However, laparoscopic approach also results in increased incidence of IH due to decreased adhesions as compared to open approach. IH through one of the mesenteric defects, can result in small bowel obstruction, ischemia, with subsequent strangulation and infarction with increased morbidity in these patients. In fact, IH is the most common cause of bowel obstruction after LGBP. The purpose of presenting this case, is to highlight the recognition of early clinical signs and symptoms associated with presentation of IH, to enable prompt decision making in surgical management of these patients.
CASE REPORT
A 42-year-old Hispanic female presented to the emergency room with a one-day history of epigastric abdominal pain, nausea and vomiting. The pain was mostly in left upper quadrant of abdomen, sharp, intermittent, without any alleviating or aggravating factors. It was associated with multiple episodes of nausea and bilious vomiting. Patient denied any fevers, chills, diarrhea, constipation and prior similar episodes. Her relevant past surgical history included Laparoscopic Roux-en-Y gastric bypass (LGBP) 13 years ago and recent robotic assisted Total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAH BSO) 10 days ago for bleeding uterine fibroids. Patient had low grade fever with tachycardia on presentation. Her abdominal examination revealed mild distension with tenderness and fullness in the epigastrium and left hypochondriac regions of the abdomen. Rectal examination was unremarkable. Her vital signs and blood work were as shown below (Tables 1 and 2). She was admitted, with plans for bowel rest, intravenous hydration, blood work and urgent CT scan of abdomen and pelvis with soluble contrast.
| Blood pressure | 136/101 |
| Pulse | 98 (per minute) |
| Temperature (oral) | 99.2 F (Fahrenheit) |
| Respiratory rate | 15 (Per minute) |
| Height | 5 feet 2 inches |
| Weight | 186 LB |
| SPO2 | 100% |
| BMI | 34.02 kg/square meters |
| Blood pressure | 136/101 |
| Pulse | 98 (per minute) |
| Temperature (oral) | 99.2 F (Fahrenheit) |
| Respiratory rate | 15 (Per minute) |
| Height | 5 feet 2 inches |
| Weight | 186 LB |
| SPO2 | 100% |
| BMI | 34.02 kg/square meters |
| Blood pressure | 136/101 |
| Pulse | 98 (per minute) |
| Temperature (oral) | 99.2 F (Fahrenheit) |
| Respiratory rate | 15 (Per minute) |
| Height | 5 feet 2 inches |
| Weight | 186 LB |
| SPO2 | 100% |
| BMI | 34.02 kg/square meters |
| Blood pressure | 136/101 |
| Pulse | 98 (per minute) |
| Temperature (oral) | 99.2 F (Fahrenheit) |
| Respiratory rate | 15 (Per minute) |
| Height | 5 feet 2 inches |
| Weight | 186 LB |
| SPO2 | 100% |
| BMI | 34.02 kg/square meters |
| Result . | Patient value . | Normal range . |
|---|---|---|
| Carbon dioxide | 19 mmol/l | 20–31 mmol/l |
| Anion gap | 18 | 7–15 |
| Urea nitrogen | 4 mg/dl | 8–26 mg/dl |
| Calcium | 8.1 mg/dl | 8.6–10.5 mg/dl |
| Red blood cells | 3.42 M/cumm | 3.73–5.09 M/cumm |
| Hemoglobin | 11.1 g/dl | 12–14.8 g/dl |
| Hematocrit | 33.3% | 36–44% |
| MPV4 | 9.3 fl | 9.4–12.3 fl |
| Albumin | 2.9 g/dl | 3.5–5.2 g/dl |
| AST | 35 IU/l | 11–34 IU/l |
| Albumin | 2.9 g/dl | 3.5–5.2 g/dl |
| Neutrophils | 88.0% | 37–85% |
| Lymphocytes | 6.6% | 12–50% |
| Neutrophils | 7.66 k/cumm | 2–7.5 k/cumm |
| Lymphocytes | 0.57% k/cumm | 1–3.5 k/cumm |
| Lipase | 11 IU/l | 5–61 IU/l |
| Prothrombin time | 14.1 secs | 11.9–14.1 secs |
| Partial thromboplastin time | 29.4 secs | 24.7–37.5 secs |
| Result . | Patient value . | Normal range . |
|---|---|---|
| Carbon dioxide | 19 mmol/l | 20–31 mmol/l |
| Anion gap | 18 | 7–15 |
| Urea nitrogen | 4 mg/dl | 8–26 mg/dl |
| Calcium | 8.1 mg/dl | 8.6–10.5 mg/dl |
| Red blood cells | 3.42 M/cumm | 3.73–5.09 M/cumm |
| Hemoglobin | 11.1 g/dl | 12–14.8 g/dl |
| Hematocrit | 33.3% | 36–44% |
| MPV4 | 9.3 fl | 9.4–12.3 fl |
| Albumin | 2.9 g/dl | 3.5–5.2 g/dl |
| AST | 35 IU/l | 11–34 IU/l |
| Albumin | 2.9 g/dl | 3.5–5.2 g/dl |
| Neutrophils | 88.0% | 37–85% |
| Lymphocytes | 6.6% | 12–50% |
| Neutrophils | 7.66 k/cumm | 2–7.5 k/cumm |
| Lymphocytes | 0.57% k/cumm | 1–3.5 k/cumm |
| Lipase | 11 IU/l | 5–61 IU/l |
| Prothrombin time | 14.1 secs | 11.9–14.1 secs |
| Partial thromboplastin time | 29.4 secs | 24.7–37.5 secs |
| Result . | Patient value . | Normal range . |
|---|---|---|
| Carbon dioxide | 19 mmol/l | 20–31 mmol/l |
| Anion gap | 18 | 7–15 |
| Urea nitrogen | 4 mg/dl | 8–26 mg/dl |
| Calcium | 8.1 mg/dl | 8.6–10.5 mg/dl |
| Red blood cells | 3.42 M/cumm | 3.73–5.09 M/cumm |
| Hemoglobin | 11.1 g/dl | 12–14.8 g/dl |
| Hematocrit | 33.3% | 36–44% |
| MPV4 | 9.3 fl | 9.4–12.3 fl |
| Albumin | 2.9 g/dl | 3.5–5.2 g/dl |
| AST | 35 IU/l | 11–34 IU/l |
| Albumin | 2.9 g/dl | 3.5–5.2 g/dl |
| Neutrophils | 88.0% | 37–85% |
| Lymphocytes | 6.6% | 12–50% |
| Neutrophils | 7.66 k/cumm | 2–7.5 k/cumm |
| Lymphocytes | 0.57% k/cumm | 1–3.5 k/cumm |
| Lipase | 11 IU/l | 5–61 IU/l |
| Prothrombin time | 14.1 secs | 11.9–14.1 secs |
| Partial thromboplastin time | 29.4 secs | 24.7–37.5 secs |
| Result . | Patient value . | Normal range . |
|---|---|---|
| Carbon dioxide | 19 mmol/l | 20–31 mmol/l |
| Anion gap | 18 | 7–15 |
| Urea nitrogen | 4 mg/dl | 8–26 mg/dl |
| Calcium | 8.1 mg/dl | 8.6–10.5 mg/dl |
| Red blood cells | 3.42 M/cumm | 3.73–5.09 M/cumm |
| Hemoglobin | 11.1 g/dl | 12–14.8 g/dl |
| Hematocrit | 33.3% | 36–44% |
| MPV4 | 9.3 fl | 9.4–12.3 fl |
| Albumin | 2.9 g/dl | 3.5–5.2 g/dl |
| AST | 35 IU/l | 11–34 IU/l |
| Albumin | 2.9 g/dl | 3.5–5.2 g/dl |
| Neutrophils | 88.0% | 37–85% |
| Lymphocytes | 6.6% | 12–50% |
| Neutrophils | 7.66 k/cumm | 2–7.5 k/cumm |
| Lymphocytes | 0.57% k/cumm | 1–3.5 k/cumm |
| Lipase | 11 IU/l | 5–61 IU/l |
| Prothrombin time | 14.1 secs | 11.9–14.1 secs |
| Partial thromboplastin time | 29.4 secs | 24.7–37.5 secs |
CT scan of her abdomen and pelvis revealed findings of previous gastric bypass with gastric pouch severely dilated and air-fluid levels measuring up to 17 cm. The excluded portion of the stomach was noted to be decompressed as a result of dilated gastric pouch. The proximal to mid small bowel was fluid-filled and severely dilated to approximately 4 cm. Distal small bowel and colon were decompressed. There was no evidence of pneumoperitoneum. Based on these findings initial diagnosis was presumed to be gastric outlet obstruction with dilated gastric pouch. Fluid hydration and nasogastric tube decompression was continued. Patient was prepared to be taken to the operation room for emergent diagnostic laparoscopy.
The intraoperative findings were not consistent with the radiological findings (Figs 1–4). As shown in the CT scan of abdomen and pelvis; multiple loops of dilated small bowel were seen, more predominantly dilated and matted bowel complex just above the spleen. The dilated bowel complex was decompressed using needle aspiration to help reduction of the bowel loops. The ileo cecal junction was then identified and traced back to the perisplenic hilar region. The trifurcation of three bowel loops (Roux limb, biliopancreatic limb and common limb) were identified with identification of ligament of Treitz. The prior gastric bypass was of ante colic- ante gastric type and Peterson’s defect was not closed. The above described bowel trifurcation had sunken postero- inferior to the spleen and had herniated through a potential space underneath the spleen and torsed around the splenic vessels and hilum. This picture was consistent with perisplenic small bowel volvulus. As noted earlier there was a massively dilated proximal small bowel segment above the spleen, displacing it caudally. Careful adhesiolysis was performed inferior to the spleen to release the segment of the trifurcation. The bowel segments once reduced were assessed and appeared viable. Of note, the spleen appeared better perfused after reduction. The remnant hernia defect of size 2 × 3 × 2.5 cm around the hilar region was then approximated using interrupted non-absorbable sutures. Enterotomy at the decompression site was closed with non absorbable suture. All potential sites of IH were re-examined and there was no evidence of any other internal herniation. Coelomic cavity was thoroughly irrigated and operation concluded successfully. Postoperative hospital stay was uneventful, and the patient was discharged home after two days.
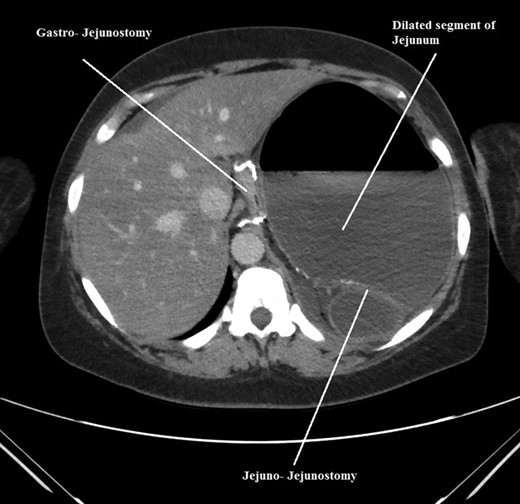
Axial CT scan of abdomen and pelvis showing, gastro-jejunostomy, Jejuno-Jejunostomy and dilated proximal small bowel segment.
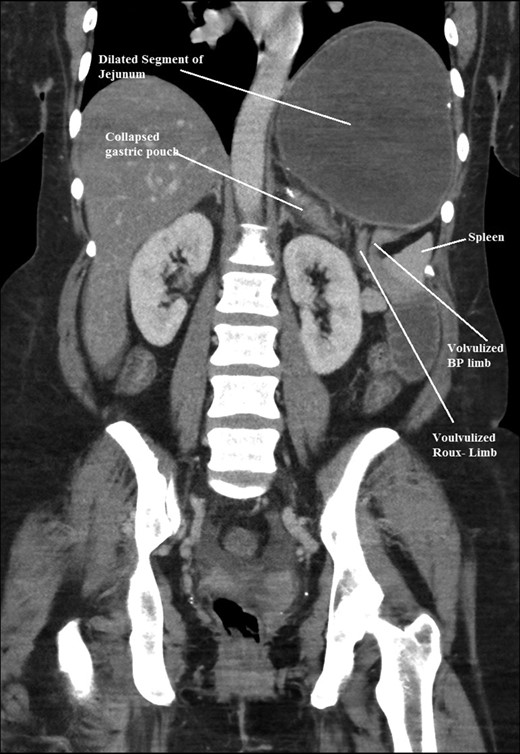
Coronal CT scan of abdomen and pelvis showing volvulized bilio- pancreatic and Roux limbs within the obstructed segments in the splenic hilar region.
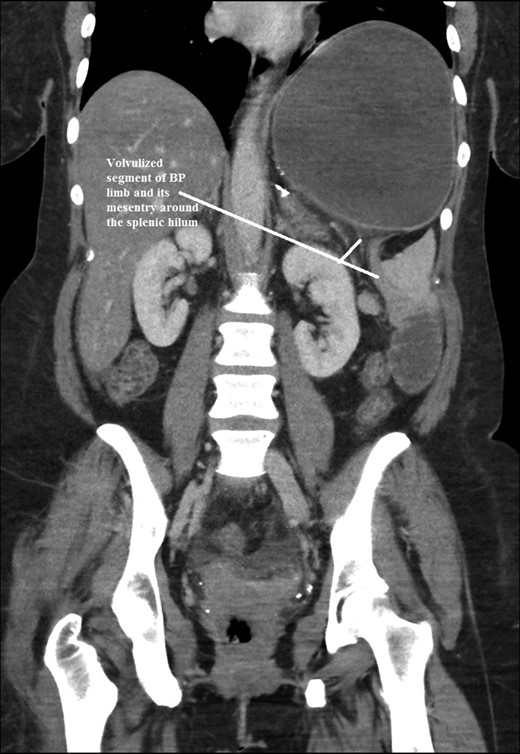
Coronal CT scan of abdomen and pelvis showing Volvulized segment of bilio-pancreatic limb and its mesentery in the spleen hilum.
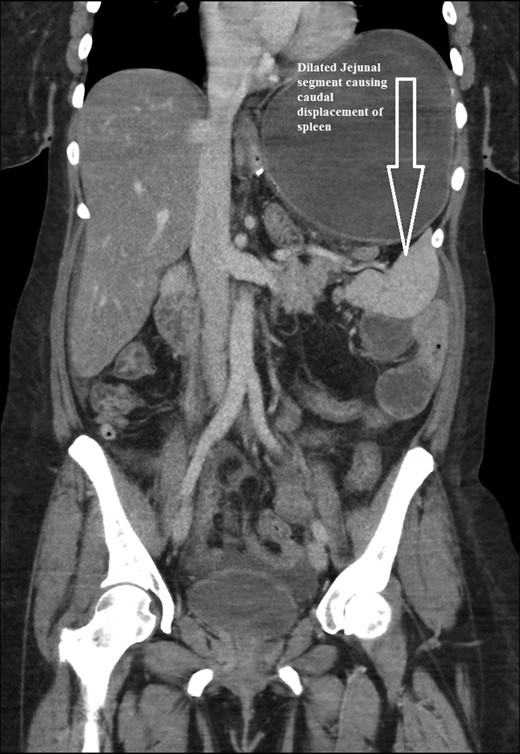
Coronal CT scan of abdomen and pelvis showing Dilated jejunal segment causing caudad splenic displacement.
DISCUSSION
The incidence of internal hernia after LRYGB varies, from 0.2% to 9% [1, 2]. Higa and Boone in his study of 2000 patients with retro colic approach showed internal hernia distribution of 67% mesocolic, 21% jejunal and 7.5% Petersen [1]. Following LRYGB the most common internal herniation is in Peterson’s space and second most common is behind jejuno-jejunostomy sites [3], others have shown mesocolic hernias are more common followed by meso jejunal and Peterson’s space hernias [1] (Figs 5 and 6). This however depends on the surgical technique and layout i.e. ante colic versus retro colic. Retro colic approach commonly leads to an additional transverse mesocolic defect hernia [1]. To prevent this, some surgeons have adopted ante colic technique. It’s reported in some studies that closing all potential hernia sites are associated with a considerable decrease in the incidence of internal herniation [2]. Despite such practice, the reduction of intra-abdominal or visceral fat after the bariatric procedures, lead to reopening of potential hernia sites [2, 4]. Studies have shown that majority of internal hernias occur after a significant (>50%) excess weight loss (EWL).
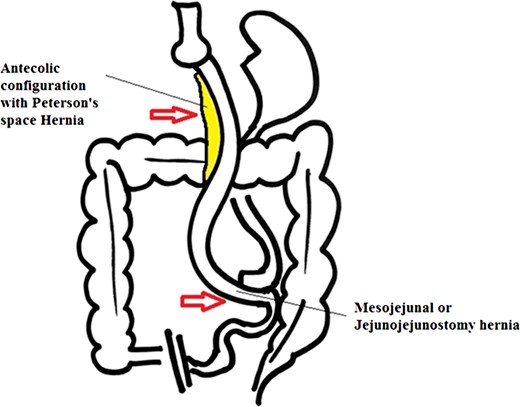
Antecolic Approach with internal hernias at two possible sites.
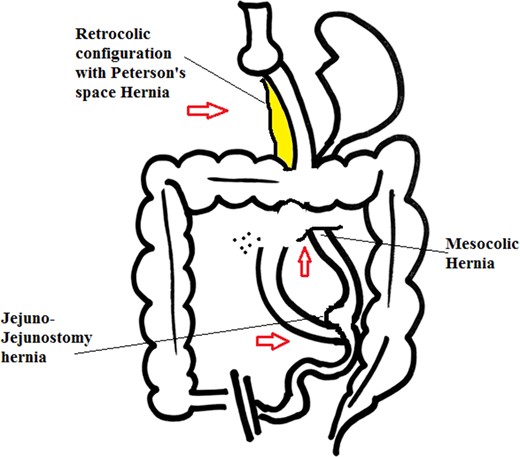
Retrocolic approach with internal hernias at three possible sites.
In post gastric bypass patients, there is no report of internal herniation occurring at unusual sites other than those described above. Our case of internal hernia clinically presented like pseudo-gastric outlet obstruction based on the CT scan findings. Only on exploration it was evident that the distended Roux limb was mis interpreted for gastric pouch. Interestingly it had displaced the spleen to a more caudad position. It is likely that the Trendelenburg position from prior hysterectomy in our patient lead to herniation around the spleen. As seen in our patient, patients with IH typically do not have clinical signs of abdominal inflammation (elevated white blood cell count, peritoneal irritation, lactic acidosis) until there is infarction or perforation of the intestine, which increases the morbidity and mortality of surgical management, as shown by Lockhart et al. [5] Physical examination is at best unreliable in obese patients.
The clinical presentation of internal hernia ranges from intermittent pain, often in the left upper abdomen through more constant abdominal pain, with or without nausea and vomiting to severe, acute abdominal pain [1]. Our patient did not have classical findings of internal herniation on the abdominal CT scan. We understand that CT scan has variable specificity and sensitivity to diagnose IH. Up to 20% of patients with IH may have CT findings negative for IH, Steele et al. [6], [1, 7, 8]. Lockhart et al. [5] found the mesenteric swirl sign to be the best single predictor of hernia with sensitivities of 61%, 78%, and 83%, and specificities of 94%, 89% and 67% for the three reviewers, respectively. This finding agreed with another group, Iannuccilli et al. [9] who looked at eight CT signs of IH which showed the mesenteric swirl sign to be most predictive of IH. Most of the radiological signs are helpful for diagnosing routine internal hernias and not necessarily in an unconventional hernia. Therefore, negative radiological results should not influence the decision to operate if clinical suspicion exists. These patients usually have rapid progression to bowel ischemia. A certain diagnosis can most often only be made by laparoscopy. Unfortunately, there is no preventive measure to prevent internal herniation and more research in this aspect is urged for in future.
CONCLUSION
The diagnosis of unconventional internal hernia after gastric bypass is a challenge and requires a high index of clinical suspicion for prompt surgical management. Diagnostic laparoscopy is paramount to aid in the diagnosis and management in these indeterminate cases.
Conflict of Interest statement
None declared.



