-
PDF
- Split View
-
Views
-
Cite
Cite
Chi-Man Yip, Huai-Pao Lee, Jui-Hsun Fu, Shuo-Hsiu Hsu, Coexistence of intracranial solitary fibrous tumor/hemangiopericytoma and right middle cerebral artery aneurysm, Journal of Surgical Case Reports, Volume 2019, Issue 1, January 2019, rjz013, https://doi.org/10.1093/jscr/rjz013
Close - Share Icon Share
Abstract
Intracranial solitary fibrous tumors are rare mesenchymal neoplasms originating in the meninges and constitute a heterogeneous group of rare spindle cell tumors that include benign and malignant neoplasms of which hemangiopericytoma is nowadays considered a cellular phenotypic variant. From literatures, the incidence of coexistence of brain tumors and intracranial aneurysms is ~0.7–5.4%. Meningioma is the most frequent tumor coexisted with intracranial aneurysms, followed by pituitary adenoma, neuroepithelial tumor, and metastatic tumor. We would like to report a case of 74-year-old man harboring a rare intracranial solitary fibrous tumor/hemangiopericytoma and an unruptured aneurysm of the right middle cerebral artery which probably the first report of these combinations in the English literature. Both lesions were treated surgically in one session with favorable outcome. Magnetic resonance angiography should be performed in patients with brain tumor preoperatively not only to visualize neoplastic vascularization but also to pick up incidental aneurysm.
INTRODUCTION
Intracranial solitary fibrous tumor is a rare mesenchymal neoplasm first described by Carneiro et al. in 1996. Hemangiopericytoma was first described by Stout and Murray in 1942 and was thought to develop from the Zimmermann’s pericytes surrounding the capillary and postcapillary venules. Both were considered as separate entities until 2016; the 2016 WHO classification of CNS tumors combined intracranial solitary fibrous tumor and hemangiopericytoma into a single disease entity because of the discovery of NAB2-STAT6 fusion using whole-exome sequencing [1, 2]. The incidence of coexistence of brain tumors and intracranial aneurysms is evaluated to be 0.7–5.4%. In the literature, meningioma is the most frequent tumor coexisted with intracranial aneurysms, followed by pituitary adenoma, neuroepithelial tumor and metastatic tumor [3]. We would like to report a case of 74-year-old man harboring intracranial solitary fibrous tumor/hemangiopericytoma and an unruptured aneurysm of the right middle cerebral artery (MCA) which probably the first report of these combinations in the English literature.
CASE REPORT
A 74-year-old man having hypertension, restless legs syndrome and parkinsonism under medical control, who suffered from headache, unsteady gait, slurred speech, decline of memory and performance for 2–3 months with progression. He had visited a local hospital and brain tumor was diagnosed after having a brain computed tomography (CT) scan. On admission, he was clear but with irrelevant and slurred speech. The muscle power of his four extremities was grade 4; Babinski’s sign and Hoffmann’s sign showed positive on bilateral sides. Other neurological examinations were unremarkable.
Brain magnetic resonance imaging (MRI) and MR angiography (MRA) revealed a large extraaxial well-circumscribed mass lesion with some calcification/hemorrhage in the right frontal–temporal convexity, abutting the skull bone, measured ~7.7 cm in maximal diameter. Post-contrast study showed good and somewhat heterogeneous enhancement with dural tail sign. Incidentally, a suspicious small aneurysm in bifurcation of the right MCA was found (Fig. 1). CT angiography (CTA) of brain was further arranged which demonstrated a wide-based saccular aneurysm in bifurcation of the right MCA, ~4.7 mm in size, abutting the tumor with dome tilting laterally, anteriorly and inferiorly (Fig. 1). Due to the significant mass effect of the brain tumor and the progressive neurological decline of the patient, surgical resection of the tumor and clipping of the aneurysm in one operation was planned.
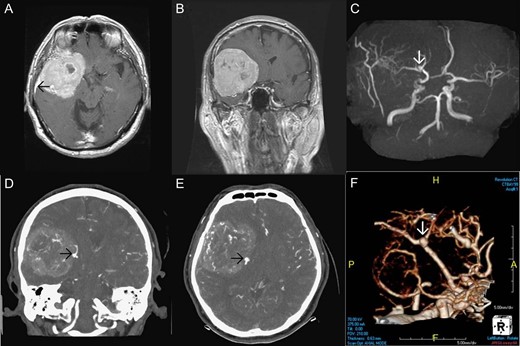
Pre-operative imaging. Axial T1 weighted image post gadolinium enhancement (A), coronal T1 weighted image post gadolinium enhancement (B) showing a large extraaxial well-circumscribed mass lesion in the right frontal–temporal convexity, abutting the skull bone with good enhancement and dural tail sign (black arrow in Fig. 1A). MRA (C) showing a suspicious small aneurysm in bifurcation of the right middle cerebral artery (white arrow). CTA of brain coronal view (D), axial view (E), 3D reconstruction (F) showing a wide-based saccular aneurysm in bifurcation of the right middle cerebral artery abutting the tumor with dome tilting laterally, anteriorly and inferiorly.
The patient underwent a right fronto-temporal craniotomy followed by flattening the right sphenoid ridge. The right middle meningeal artery and the right orbito-meningeal artery were exposed, coagulated and cut extradurally. Opened the dura and dissected the tumor along the arachnoid plane. During the procedure of tumor removal, the right internal carotid artery, the M1 segment of the right MCA, the aneurysm at the bifurcation of the right MCA were exposed, then the aneurysm was clipped. The procedure of tumor removal proceeded and Simpson grade 2 resection of the tumor was achieved. Grossly, the tumor was encapsulated, adherent to the dura of the right sphenoid ridge. The consistency of the tumor was elastic to firm and the vascularity of the mass was very high.
The weight of the resected specimen was 133 g. The tumor consisted of spindle cells in fascicular and storiform architecture with collagen fibers invested in between tumor cells, and round oval nuclei in haphazard pattern with limited intervening stroma. Some staghorn vessels were identified. Mitosis was present, up to 4/10 HPF (high power field). No tumor necrosis was identified. The neoplastic cells show immunoprofile of STAT6(+), CD34(+), EMA(+, weak) and S100(−). Intracranial solitary fibrous tumor/hemangiopericytoma grade 2 was diagnosed based on the morphology of the tumor cells and the result of immunohistochemical stains (Fig. 2).
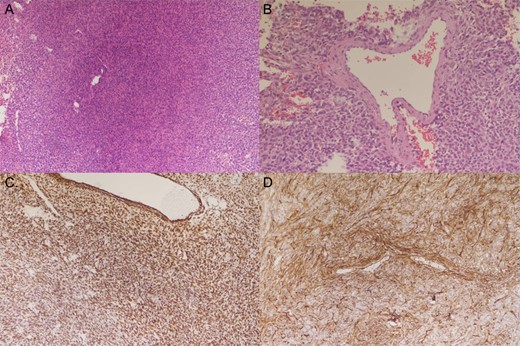
Histology of the specimen. Tumor HE stain 100× (A) showing spindle cells in fascicular and storiform architecture with collagen fibers invested in between tumor cells, and round oval nuclei in haphazard pattern with limited intervening stroma. Tumor HE stain 200× (B) showing staghorn vessel. Tumor STAT6 stain (C) and CD34 stain (D) showing positive staining.
The patient recovered well after surgery and can achieve independent life. His early post-operative CTA of brain (Fig. 3) showed the complete removal of the tumor and the complete surgical clipping of the aneurysm without residual lesions. Post-operative adjuvant radiotherapy was arranged since intracranial solitary fibrous tumor/hemangiopericytoma grade 2 is locally aggressive.
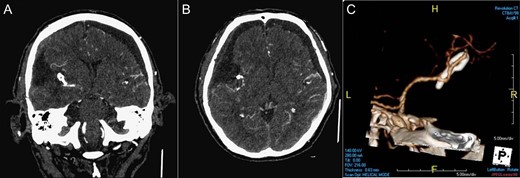
Post-operative imaging. CTA of brain coronal view (A), axial view (B), 3D reconstruction (C) showing the complete removal of the tumor and the complete surgical clipping of the aneurysm without residual lesions.
DISCUSSION
The exact cause for the coexistence of brain tumor and intracranial aneurysm remains unclear. In the literature, the incidence of coexistence of brain tumors and intracranial aneurysms is ~0.7–5.4%. Meningioma is the most frequent tumor coexisted with intracranial aneurysms, followed by pituitary adenoma, neuroepithelial tumor and metastatic tumor. The treatment strategy should be designed based on the conditions of tumor and aneurysm, locations of both lesions and pathologic nature of tumor. Magnetic resonance angiography should be performed in patients with brain tumor preoperatively not only to visualize neoplastic vascularization but also to pick up incidental aneurysm which may cause disaster if undetected [3].
Intracranial solitary fibrous tumor and hemangiopericytoma have overlapping features in clinical, radiological, histological and immunohistochemical fields. Since 2016, they have been considered as a single disease entity because of the discovery of NAB2-STAT6 fusion using whole-exome sequencing [1, 2]. Complete surgical resection with neurological function preservation is the goal of treatment. Radiotherapy can be administered as adjuvant therapy for cases showing an aggressive phenotype or not treated with gross-total resection [1].
We report a patient harboring a rare combination of intracranial solitary fibrous tumor/hemangiopericytoma and an unruptured aneurysm of the right MCA successfully treated in one surgery. Pre-operative magnetic resonance angiography helped us to avoid inadvertent rupture of the coexisted aneurysm during the operation.
CONFLICT OF INTEREST STATEMENT
We report no conflicts of interest.
REFERENCES
- phenotype
- magnetic resonance angiography
- intracranial aneurysm
- aneurysm
- brain tumors
- cancer
- heterogeneity
- hemangiopericytoma
- meningioma
- middle cerebral artery
- neoplasm metastasis
- neoplasms, neuroepithelial
- brain
- meninges
- neoplasms
- pituitary adenoma
- solitary fibrous tumor
- aneurysm, middle cerebral artery



