-
PDF
- Split View
-
Views
-
Cite
Cite
Jessica G Y Luc, Holger Buchholz, Daniel H Kim, Roderick G G MacArthur, Left ventricular assist device for ventricular recovery of anabolic steroid-induced cardiomyopathy, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy221, https://doi.org/10.1093/jscr/rjy221
Close - Share Icon Share
Abstract
Herein we report a case of a 26-year-old gentleman with severe cardiomyopathy likely secondary to anabolic-androgenic steroid (AAS) abuse who received a HeartMate II (Abbott Laboratories, Abbott Park, IL) left ventricular assist device (LVAD) for rapidly deteriorating heart failure with hemodynamic compromise. Following 18 months on LVAD support, excellent recovery of ventricular function was achieved to allow for LVAD discontinuation. Given that active substance abuse is a contraindication to heart transplantation, few options remain for patients with AAS induced heart failure. Our case demonstrates that LVAD therapy can be an important intervention for bridging to candidacy, recovery or destination therapy.
INTRODUCTION
Anabolic-androgenic steroid (AAS) abuse has increased in recent years [1]. Supraphysiological doses and high-frequency usage of AAS have been linked to adverse cardiovascular effects [1]. Active substance abuse is a contraindication to heart transplantation and patients are required to have a 6-month period of abstinence prior to listing for heart transplantation [2]. As such, left ventricular assist device (LVAD) therapy is an important intervention for bridging to candidacy, recovery or, if they are unable or unwilling to abstain from substances, as destination therapy. We report a case of severe cardiomyopathy likely secondary from AAS abuse that was managed with implantation of a Centrimag Centrifugal Pump (Abbott Laboratories, Abbott Park, IL) followed by a HeartMate II LVAD (Abbott Laboratories, Abbott Park, IL) with optimization of medical therapy and eventual successful device discontinuation.
CASE PRESENTATION
A 26-year-old previously healthy man presented to the emergency department with shortness of breath, hemoptysis and a presumed diagnosis of worsening pneumonia resistant to antibacterial treatment. Computed tomography of the chest revealed bilateral extensive airspace opacities. Differential diagnoses included hemorrhage, cryptogenic organizing pneumonia, cardiogenic edema and atypical infection. The patient had a history of AAS abuse over the past 3 years with testosterone, trenbolone and primobolan as well as human growth hormone, alcohol, cocaine and 3,4-methylenedioxy-methamphetamine. Past medical and family histories were unremarkable, specifically with no familial cardiac issues.
Echocardiography revealed an estimated left ventricular ejection fraction (LVEF) <10%. A severely dilated and impaired left ventricle and mildly dilated right ventricle were confirmed with MRI. He deteriorated rapidly requiring intubation for respiratory failure as well as multiple vasopressors and inotropes for cardiogenic shock. Furthermore, he suffered from multiple asystolic cardiac arrests requiring cardiopulmonary resuscitation and acute renal failure requiring initiation of PRISMA.
Heart transplantation and mechanical circulatory support were considered. Given his critical condition and active substance abuse, he was deemed unsuitable for transplantation at this time. Temporary circulatory support with the Centrimag Centrifugal Pump was urgently inserted off pump through an upper hemisternotomy and left anterolateral thoracotomy as a bridge to decision. Following recovery of neurological and end-organ function, he was converted to a durable device (HeartMate II LVAD) as a bridge to candidacy, recovery or as destination therapy.
The HeartMate II LVAD was implanted in the usual fashion. Chest radiographs on admission and following implantation of LVAD are shown in Fig. 1. LVAD core pathology revealed cardiomyocyte hypertrophy, patchy myocyte death and histiocytic inflammatory reaction consistent with AAS induced heart failure [1, 3]. Postoperatively, he made gradual and steady improvement and was discharged home on postoperative day (POD) 34. He was connected with addictions counseling, rehabilitation and support to which he was compliant with. He was also maintained on aggressive medical therapy for heart failure including beta-blockers, afterload reduction with an angiotensin-converting enzyme inhibitor (ACEi), an angiotensin II receptor blocker (ARB) as well as aldosterone antagonists.
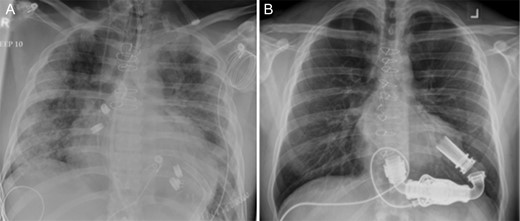
Chest radiographs (A) on admission, and (B) following implantation of a HeartMate II Left Ventricular Assist Device.
Following 18 months in the community on LVAD support with normal quality of life, there was demonstration of recovery in ventricular function with a LVEF of 55% (vs. initial <10%), left ventricular internal diameter end diastole 4.2 cm (vs. initial 7.4 cm) and systole of 2.6 cm (vs. initial 6.9 cm). With a subsequent weaning trial, performed as per the literature [4], demonstrating good hemodynamic and functional parameters, a decision was made to proceed with device discontinuation. Rather than a definitive LVAD explantation and to minimize trauma to the recovered myocardium, the LVAD was discontinued by removing the driveline in its entirety and occluding the outflow graft [5]. This allowed the device to be left in-situ and leaves a natural orifice to re-implant a new device at a later date should that be necessary with recurrence of heart failure in the long-term [6]. Heart function remained excellent post-LVAD discontinuation (LVEF >60%) and the patient was discharged on POD 7 on full heart failure management.
DISCUSSION
AAS are a family of hormones that include the natural male hormone testosterone as well as its synthetic relatives, all of which exhibit anabolic and androgenic properties. Individuals use AAS to increase strength, lean body mass and improve physical appearance with lifetime prevalence of AAS use among male adolescents in Western countries of between 1 and 5% [1]. Adverse cardiovascular effects of AAS include left ventricular hypertrophy, impaired left ventricular function, arterial thrombosis, pulmonary embolism and myocardial infarction without significant coronary artery disease [1].
Potential mechanisms of cardiovascular toxicity of AAS include direct myocardial injury, atherogenic, thrombotic, hemostasis and vasospastic effects [7]. Based on rat and pathological studies, the cardiotoxic effects of AAS abuse on human cardiac muscle are thought to cause left ventricular hypertrophy leading to impaired diastolic function, eventually systolic dysfunction and dilatation [3].
Literature regarding the potential for ventricular recovery or reversibility of AAS induced cardiomyopathy after discontinuation of AAS remains sparse. Reports of partial ventricular recovery following withdrawal of AAS and beginning of medical treatment for heart failure have been described [8]. Controversy regarding ventricular recovery may be due to individual susceptibility to the toxic effects of AAS that underlie the variable response in different patients.
Heart transplantation is widely recognized as a successful modality to treat end-stage cardiomyopathy, but is limited by the lack of donor organs [2]. Active substance abuse is regarded as a contraindication for heart transplantation [2]. Thus, LVADs can offer an opportunity for medical stabilization, substance abuse rehabilitation and subsequent re-evaluation of candidacy. For those that remain ineligible for transplantation, this therapy presents a viable long-term mechanical circulatory therapy and in some cases, potential ventricular recovery and LVAD discontinuation for this subset of patients. Patients with AAS abuse would also benefit from patient and family education as well as multidisciplinary team involvement including addictions counseling and rehabilitation to achieve abstinence of substance use.
The present report demonstrates that severe cardiomyopathy likely secondary from AAS abuse can be successfully supported with a durable LVAD with excellent recovery of ventricular function. Our report also highlights that AAS use should be considered in the differential diagnosis of left ventricular systolic dysfunction. Improved education and awareness of complications of AAS may lead to better recognition and prompt treatment of their potentially lethal effects.
CONFLICT OF INTEREST STATEMENT
H.G. and R.G.G.M. are consultants for Abbott Laboratories. All other authors do not have any conflicts of interest to disclose.
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.



