-
PDF
- Split View
-
Views
-
Cite
Cite
Rickesh B Karsan, Bilal Al-Sarireh, Ruptured pseudoaneurysm of an unnamed vessel as complication of chronic pancreatitis, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy194, https://doi.org/10.1093/jscr/rjy194
Close - Share Icon Share
Abstract
Pseudoaneurysms in the celiac territory are mostly managed conservatively. Endovascular thromboembolisation with or without stenting is currently the standard treatment with good outcome. We present a case of a patient with chronic pancreatitis who initially underwent endovascular treatment for presumed splenic artery pseudoaneurysm and subsequently required surgical intervention for complicated unnamed artery pseudoaneurysm. Radiological diagnosis was instrumental in guiding the ensuing surgical procedure and the patient made a good postoperative recovery.
INTRODUCTION
Chronic pancreatitis increases the risk for visceral pseudoaneurysms due to the chronic inflammation process. Haemorrhage is the most common complication and the presenting symptoms include abdominal pain, anaemia, upper gastrointestinal (GI) bleeding and hypovolaemic shock. Gastric artery pseudoaneurysms are less common (5%) compared to splenic (40%) and gastroduodenal pseudoaneurysms [1].
Embolization success rates in the literature range between 79 and 100% and post embolization mortality rates between 6 and 33% [2].
CASE REPORT
We present a case of pseudoaneurysm in a 40-year-old man with long-standing chronic alcohol pancreatitis.
He presented to the local tertiary referral centre complaining of chest pain and the normal ECG, cardiac markers and chest X-ray suggested a non-cardiac cause for the pain. He remained haemodinamically stable and was further investigated with an abdominal ultrasound (USS) and a contrast enhanced computed tomography (CT). The latter demonstrated a 5 × 3.8 cm2 pseudoaneurysm posterior to the stomach and superior to the body of the pancreas but of unclear origin. It also showed a large subcapsular splenic haematoma measuring 10.8 × 7.7 cm2 and a perisplenic collection (Fig. 1).
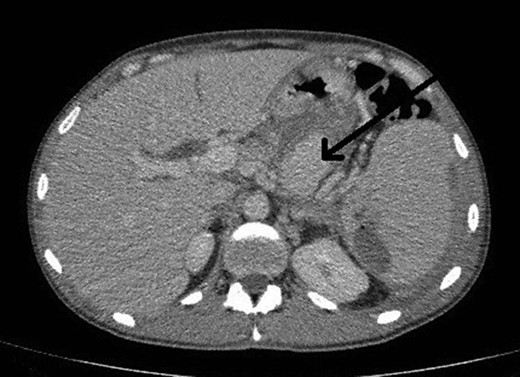
CT abdomen image shows flow in pseudoaneurysm (arrow) posterior to the stomach and the subcapsular splenic haematoma.
At this point urgent angiography for embolization was planned. In the meantime increasing complaints of abdominal pain prompted a review and optimization of the medication. In preparation for the procedure the clotting profile was checked and the patient gave written informed consent.
Selective angiogram using the Seldinger technique via left brachial approach to visualize the celiac trunk and superior mesenteric artery branches failed to show the pseudoaneurysm leading to the conclusion that possibly it had a different origin not accessible by angiography (Fig. 2).
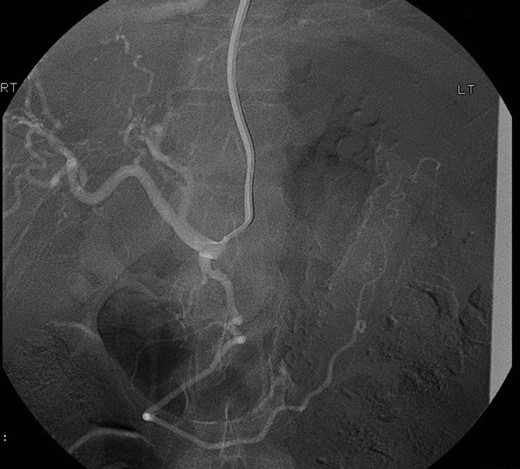
Selective angiogram of the celiac trunk failing to demonstrate the pseudoaneurysm.
The next step was an endoscopic ultrasound guided thrombin injection. This was carried out and 2 ml of thrombin was injected into the pseudoaneurysm to good effect with no immediate complications. Also, percutaneous drainage of the perisplenic collection was carried out, where 400 ml of pus was drained. A follow-up duplex ultrasound scan confirmed that there was no flow in the pseudoaneurysm (Fig. 3).
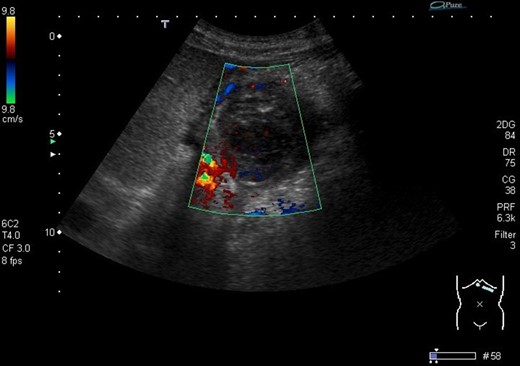
Ultrasound image demonstrates the thrombosed pseudoaneurysm 24 h post thrombin injection.
At this point it looked like the therapeutic approach was successful and on the ward the patient appeared clinically stable.
However, 4 days after the ultrasound guided injection, the patient collapsed on the ward with haematemesis and hypovolaemic shock. He was resuscitated initially with intravenous fluids and transfusion of blood products and remained alert with no abdominal signs. An emergency gastroscopy showed a 1.5 cm gastric ulcer on the posterior gastric wall with a large clot in the upper stomach but no active bleeding. A repeat CT abdomen demonstrated persistent flow in the pseudoaneurysm (Fig. 4).
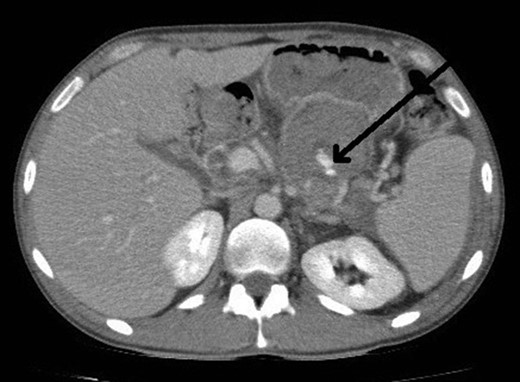
CT abdomen image post thrombin injection shows flow in the pseudoaneurysm (arrow).
3D reconstruction images of the CTA revealed that the pseudoaneurysm was localized on an unnamed vessel branching directly from celiac trunk (Fig. 5). This deemed not suitable for endovascular embolization due to its size.
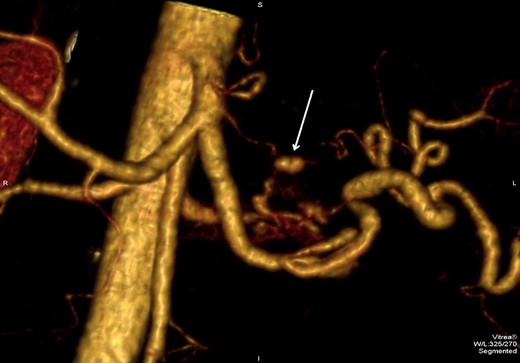
3D CTA reconstruction demonstrates the pseudoaneurysm (arrow) originating from the unnamed vessel.
As a result the decision was made to proceed with laparotomy. The procedure involved evacuating the haematoma, and over sowing the bleeding vessel and a subtotal gastrectomy with roux-en-y gastro-jejunostomy.
The patient returned to intensive care postoperatively where his progress was delayed by hospital acquired pneumonia, episodes of failed extubation, agitation and moderate malaena managed with blood transfusion. He returned to the ward and was eventually discharged after 5 weeks of overall hospital stay.
At an outpatient review 6 weeks after discharge the patient was generally well but complained of ongoing diarrhoea and failure to gain weight. This was attributed to chronic pancreatitis and managed with enzyme supplements and high calorie additives.
DISCUSSION
The pathogenesis of pseudoaneurysms in chronic pancreatitis is related to repeated episodes of pancreatic inflammation that leads to disruption of the duct and ductule system causing fluid exudation into the peripancreatic area. The enzymes in the fluid digest and erode the adjacent blood vessels leading to aneurismal dilatation and necrosis [3]. Necrosis can cause rupture into a pre-existing pseudocyst or haemorrhage into the abdominal cavity, retroperitoneum, GI tract, pancreatic ducts, biliary tree or bowel mucosa after erosion of the adjacent bowel [4].
The most common pattern of bleeding from pseudoaneurysms is into a pseudocyst and thus into the gastrointestinal tract [5] with intermittent episodes possible explained by the fact that the increased pancreatic duct pressure leads to thrombosis of its communication with the pseudoaneurysm followed by duct decompression with clot lysis thus repeating the cycle. Less commonly the bleeding can occur through fistulation of the pseudoaneurysm into the GI tract.
Angiography is currently the gold standard and is very important in identifying the pseudoaneurysms too small to be diagnosed with other types of imaging [6]. It is vital not only for diagnosis but also for therapeutic embolization which has similar outcomes compared to surgery but can include more elderly and unwell patients.
The treatment of pancreatic pseudoaneurysms is divided between endovascular and surgical techniques. Visceral artery embolization is considered by some to be the first line treatment for bleeding pseudoaneurysms [7] and has been used alone for the definitive treatment of uncomplicated pseudoaneurysms. This can be used as an interim measure until definitive treatment in unstable patients. Limitations are imposed when the feeding vessel(s) cannot be demonstrated, not accessible or too small.
Surgery is indicated in cases with negative angiography, when the vessel(s) is not amenable to embolization or when re-bleeding occurs after successful embolization. Mortality rates with surgical treatment vary between 10 and 50% according to the literature and are related to initial hemodynamic state, transfusion requirement and category of pancreatitis.
CONCLUSION
We presented a case of a ruptured pseudoaneurysm in an unnamed vessel as a complication of chronic pancreatitis managed surgically after unsuccessful embolization therapy.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
None.
FUNDING
None.



