-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel Lim, Raymond Kostin, Intralobar pulmonary sequestration associated with Bochdalek hernia: first reported case in an adult male and literature review, Journal of Surgical Case Reports, Volume 2018, Issue 8, August 2018, rjy211, https://doi.org/10.1093/jscr/rjy211
Close - Share Icon Share
Abstract
Pulmonary sequestrations (PS) are rare congenital malformations that can be difficult to diagnose. PS have no connection with the bronchial tree and are vascularized through an aberrant artery mostly arising from descending thoracic or abdominal aorta. The standard diagnostic method is the computed tomography angiography and delayed diagnosis can lead to recurrent pneumonia and hemoptysis. Herein, we have a case of a patient with an intralobar sequestration associated with a Bochdalek hernia. The diagnosis was delayed in this case as with many other similar case reports because details of the patient’s history were overlooked.
INTRODUCTION
Pulmonary sequestrations (PS) are congenital pulmonary malformation consisting of non-functional lung tissue that have no communication with the tracheobronchial tree. The incidence of PS is ~0.29 accounting for 0.15–6.4% of all congenital lung anomalies [1]. The incidence in the adult population has not been reported as it is an even more rare of a finding. PS manifest by non-specific respiratory and cardiovascular features such as cough, purulent sputum, wheezing, chest tightness, fever, regurgitation, cyanosis and hemoptysis. Thus, PS can be misdiagnosed with many other clinical conditions with similar features such as pneumonia, tuberculosis, pulmonary cyst, lung abscess, pulmonary tumor and asthma [2]. There are mounting research papers reporting an association between Bochdalek hernias and PS [3, 4]. A Bochdalek hernia is the most common congenital diaphragmatic hernia affecting 1/2500 cases and originally formed through a defect in the pleuroperitoneal structure during embryogenesis [5]. Although PS has been associated with CDH, this is the first case of intralobar PS (ILPS) associated with a Bochdalek hernia.
CASE PRESENTATION
We report a case of 48-year-old Caucasian non-smoking male, admitted to the chest service for worsening pneumonia-like symptoms for the past 3 weeks (generalized malaise, productive cough and intermittent fevers and chills) despite receiving intravenous antibiotics. Chest examination revealed significant expiratory wheezing and crackles at the right lung base. The admission chest x-ray (Fig. 1A) which was subsequently confirmed with a computed tomography (CT) scan showed a large right hydropneumothorax of the right lower lung lobe (Fig. 1B). He had no history of exposure to asbestosis or tobacco products. However, 5 years ago, the patient experienced similar symptoms with a homogeneous CT scan for which he underwent a video-assisted thoracoscopic surgery (VATS) with partial decortication, for what they diagnosed to be an empyema. Based on his history and CT findings, the potential diagnosis of recurrent empyema emerged. He was thus started on broad-spectrum antibiotics and prepared for decortication the next day.
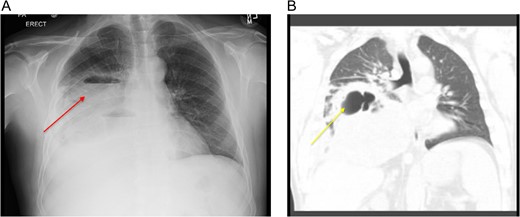
(A) CXR showing a large loculated right hydropneumothorax of the right lower lung lobe. (B) Computed tomography scan showing a large loculated right hydropneumothorax of the right lower lung lobe.
Because of the extent of this empyema, we did not carry out a video-assisted approach and proceeded immediately to a thoracotomy. We performed a thoracotomy with decortication and drainage of empyema with aspiration of ~300 ml of frank pus. The patient had no history of bleeding disorders; however, the intraoperative blood loss approached two liters. Postoperatively, the patient was very fatigued, unable to be weaned of supplemental oxygen, intermittently spiking fevers and his white blood cells (WBCs) trended in an upward direction. On the fifth post-operative day, CT scan was redone and revealed a persistent large loculated fluid collection in the right and middle lobes of his lung (Fig. 2). Interventional radiology was consulted and a 12 Fr catheter was placed into this fluid collection which drained out dark brown foul-smelling fluid. Because of the consistency of the drainage, we suspected that this patient developed an infected hematoma. On the seventh day, his condition continued to deteriorate and required pressure support. We subsequently took him back to the operating room for decortication and drainage of a suspected hematoma. In the operating room, we found two distinct large abscess pockets in the right lower lobe which we broke up and drained completely. Of note, we again encountered a lot of intraoperative bleeding loss.
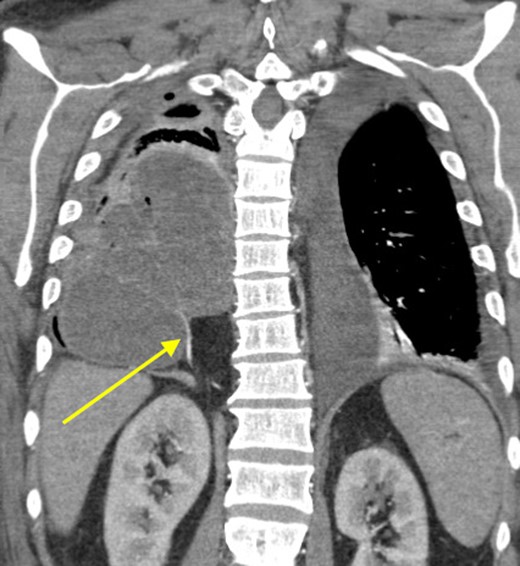
Coronal view showing persistent empyema and anomalous arterial supply into sequestration.
At that time, we started to wonder if this patient had ILPS, which prompted us to scrutinize his prior CT scans. Surprisingly, we noticed an anomalous arterial supply from the right renal artery that traversed through his Bochdalek hernia and into the sequestration (Figs 2 and 3). Two days after the operation, the patient was extubated and his clinical course was relatively benign. Also, no pathogens were detected by microbiological analysis of the intraoperative cultures and bronchial lavage. Pleual biopsies from the first operation just showed some acute fibrinous pleuritis with calcium. One week later, his mental status recovered at baseline, he was breathing without supplemental oxygen and his WBCs count had normalized. The patient was discharged to rehab with follow up in the clinic to discuss surgical resection of the ILPS in order to prevent recurrent symptoms.
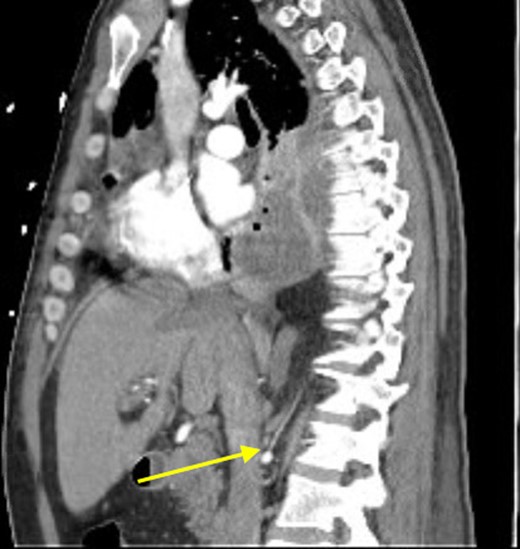
Sagittal view showing anomalous bloody supply originating from proximal right renal artery.
DISCUSSION
PS are congenital non-functional lung tissue, difficult to diagnose and rarely reported in the literature. To the best of our knowledge, this is the first case of ILPS associated with Bochdalek hernia in an adult. The PS is divided anatomically into ILPS and extralobar PS (ELPS) sequestration. The ILPS is much more common, accounting for 75–85% of all PS and it is blended in the normal lung tissue while the ELPS is separated from the normal lung by its distinct visceral pleura [1, 6]. Another main difference is the venous drainage, where the ILPS drains into the pulmonary circulation and ELPS drains into the systemic circulation [1, 6].
Since PS may be misdiagnosed with many other clinical conditions, our clinical suspicion was delayed [2]. A diagnostic clue for PS is attained through CT scan and angiography that identify the parenchymal and vascular structures of the PS [7]. Similar to our case when we realized the arterial supply coming from the right renal artery into the sequestration. It is known that pulmonary arteries normally supply the lung; however, the PS receives its blood supply through an aberrant artery, mostly arising from thoracic (75%) or abdominal aorta (20%). Also, there are few reports of PS with blood supply via intercostal, subclavian and internal thoracic arteries [6, 8]. These high-pressure systemic arteries make the patient of ILPS at risk of uncontrolled hemorrhage during the operations, such as our patient who had no bleeding disorders and lost a large amount of blood during the operation. We believe that earlier diagnosis is critical to lessen morbidity and mortality for this rare congenital lung malformation. Such as in our patient, the delayed diagnosis and management lead to recurrent pneumonia-like symptoms and the patient having to undergo another thoracotomy.
Lobectomy of the affected lung is the definitive management for symptomatic and asymptomatic patients with ILPS. Segmentectomy can be an alternative option, but it carries the risk of incomplete resection [9]. The surgery can be carried out through VATS which is a less invasive approach and a substantial method for resection of PS. Although the recurrent infection and fibrosis may hinder the use of VATS, promising outcomes were reported in a retrospective analysis of 14 thoracoscopically treated subjects, with only one case requiring conversion to thoracotomy due to bleeding [10].
CONCLUSION
PS are rare and the diagnosis may be challenging and delayed due to non-specific clinical features. Not only was this an interesting case where we encountered the presence of two unique congenital anomalies in and adult, but it was a humbling experience to have misdiagnosed our patient as we overlooked certain parts of his clinical history. From our report, we believe that ILPS should be considered in the differential diagnosis of recurrent pneumonia-like symptoms in young otherwise healthy adults. Prior to surgical intervention, a CT angiogram should be carefully reviewed with a radiologist to detect any aberrant artery or arteries supplying the sequestration in order to avoid any intraoperative complications.
CONFLICT OF INTEREST STATEMENT
None declared.



