-
PDF
- Split View
-
Views
-
Cite
Cite
Jodie Trautman, Steven J Craig, Gastric inlet obstruction from oesophageal cancer with internalized gastric band: a worrisome outcome?, Journal of Surgical Case Reports, Volume 2018, Issue 6, June 2018, rjy118, https://doi.org/10.1093/jscr/rjy118
Close - Share Icon Share
Abstract
Band erosion is one late complication of laparoscopic adjustable gastric banding (LAGB), with the reported incidence between 1% and 28%. Far less common is oesophageal adenocarcinoma after LAGB, with only three cases previously described. Here we report a single case of complete gastric inlet obstruction with oesophageal adenocarcinoma and complete internalization of a gastric band 19 years after its placement.
INTRODUCTION
Laparoscopic adjustable gastric banding (LAGB) is an effective weight loss procedure that is often chosen because of the minimally invasive nature of the operation and potential for reversal. Band erosion is an uncommon and usually late complication with a highly variable incidence, reported to be anywhere between 1% and 28% [1–3]. Rarely, it can present as a cause of gastro-intestinal obstruction. Oesophageal adenocarcinoma has been reported in only six cases after a bariatric procedure, three of which were post LAGB [4–7]. The case we present is of complete gastric inlet obstruction, oesophageal adenocarcinoma and complete internalization of a gastric band 19 years after its placement.
CASE REPORT
A 65-year-old female presented with a 6-week history of vomiting. This had progressed to complete intolerance of all solid and liquid oral intake, except for small sips of water. The patient also described epigastric discomfort followed by a band-like pain around the circumference of her thorax, at the level of the epigastrium. Her LAGB had been performed at another institution 19 years prior. There was subsequent tightening of the gastric band three years following initial placement. Due to geographical relocation of the patient, there was no formal follow-up. The patient reported acceptable weight loss and maintenance, with no adverse symptoms. She had an unremarkable abdominal examination. Computed tomography imaging identified oral contrast pooled above the level of the laparoscopic band, at a much lower level in the stomach than expected. The small and large bowel were collapsed and almost gasless, suggesting band slippage and absolute obstruction (Fig. 1a and b). Exploratory laparoscopy and removal of the gastric band was performed on the following day.
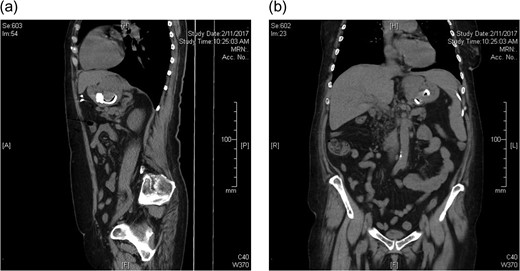
CT abdomen prior to laparoscopic gastric band removal (a) sagittal and (b) coronal.
Extensive adhesiolysis was required intra-operatively to mobilize the left lobe of the liver from the stomach. The tube of the adjustable gastric band could be seen tracking into the stomach wall near the greater curvature, with the band completely internalized in the stomach. Dissection along the gastric tube into the lumen of the stomach allowed removal of the internalized gastric band with an endocatch bag. A 3-cm gastric defect on the anterior stomach wall was closed with laparoscopic sutures. Gastroscopy was then attempted during the same procedure. At the gastro-oesophageal junction, there was an obstructing lesion presumed to be scar tissue and fibrosis that had surrounded the internalized gastric band, however it was also biopsied to exclude malignancy. The obstruction could not be traversed with the endoscope. There was a presumed small patency as gastric insufflation was possible via the gastroscope. Given this gastric inlet obstruction, a decision was made to place a surgical gastrostomy tube for gastric decompression and to allow enteral nutrition.
The patient was discharged on the sixth day post operatively. Five weeks following the initial procedure, the patient underwent repeat gastroscopy. The obstructing lesion was not reduced in size and still could not be passed by the gastroscope. Histopathology of the biopsies taken was that of a low grade invasive adenocarcinoma. Staging investigations with computed tomography and positron emission tomography identified the distal oesophageal lesion extending in to the stomach, along with bilateral lung nodules likely inflammatory in nature however potentially early metastases. The patient received neoadjuvant chemotherapy and radiotherapy prior to operative resection.
DISCUSSION
The risk of cancer after a bariatric procedure is unclear. Obesity itself is a risk factor for Barrett’s metaplasia and oesophageal adenocarcinoma by mechanisms that may include increased gastro-oesophageal reflux disease (GORD), hormonal, insulin and dietetic factors [5, 8]. This is one reason routine endoscopy is part of the pre-operative work up for bariatric surgery. On the other hand, the high incidence of GORD and oesophagitis post bariatric surgery has the potential to translate into Barrett’s metaplasia, thus increasing the risk of oesophageal carcinoma in the long term. One study identified Barrett’s metaplasia in 28% of patients with symptomatic reflux who received endoscopy before revisional surgery of a restrictive procedure [9].
However, subsequent cancer of the oesophagus or stomach after bariatric surgery has to date been reported in less than 25 cases [5]. Oesophageal adenocarcinoma is reported in only six cases, three of which were after LAGB, two at 2 years and one at 8 years after placement [6–8]. Another two cases of adenocarcinoma at the gastro-oesophageal junction were at 14 and 21 years post-Roux-en-Y Gastric Bypass, and one 16 years post-Vertical Banded Gastroplasty [4]. It is possible that these cases of oesophageal carcinoma represent pathology missed during pre-operative LAGB screening, given the early timeframe of presentation, and moreover a potentially delayed diagnosis due to post-operative band side effects. De Roover et al. [5] recommends endoscopic surveillance in patients more than 15 years after gastric surgery, or those symptomatic with GORD due to the high rate of Barrett’s metaplasia that has been reported.
While only several cases of oesophageal cancer have been reported after LAGB, this may reflect the time interval of follow-up. This may be particularly relevant in the Asia-Pacific region, where LAGB was the bariatric procedure of choice until only 8 years ago [10]. In the case we present, the patient was entirely asymptomatic until 19 years after the original placement of the LAGB. There is prospect that future cases of oesophageal adenocarcinoma may become evident in patients many years after LAGB and we suggest a role for endoscopic surveillance of patients with symptomatic GORD after LAGB, particularly as the time interval since placement increases.
Conflict of interest statement
None declared.



