-
PDF
- Split View
-
Views
-
Cite
Cite
Rhona H Hurley, Michael McCormick, Mohamad Elhassan, Gary Nicholson, Gastrointestinal stromal tumour as a rare association with neurofibromatosis type 1, Journal of Surgical Case Reports, Volume 2018, Issue 2, February 2018, rjy017, https://doi.org/10.1093/jscr/rjy017
Close - Share Icon Share
Abstract
Gastrointestinal stromal tumours (GIST) are rare tumours of mesenchymal origin. These can be associated with neurofibromatosis type 1 (NF1), which is an autosomal dominant disorder. The prevalence of GIST in NF1 is estimated at 3.9–25%. This paper describes the presentation of a GIST arising from the jejenum in a 75-year-old lady with NF1, who presented with gastrointestinal bleeding. This was diagnosed by CT angiography. She was managed with laparotomy, with resection of small bowel, and an ischaemic segment of large bowel with two primary anastomoses. Pathology showed GIST of spindle cell type (Figs 3 and 4), 90 mm in size, with complete local excision. The patient was discharged on the eighth post-operative day and is currently undergoing regular clinic follow-up after multidisciplinary team meeting discussion.
INTRODUCTION
A 75-year-old lady presented with melaena and per rectum bleeding and was found to have a gastrointestinal stromal tumour (GIST) of the small intestine, on a background of neurofibromatosis type 1 (NF1). During the same admission, this was successfully resected with two primary anastomoses. The patient was discharged home on the eighth post-operative day.
This is a rare presentation of GIST in association with NF1. Although this is a known association, cases are still rare in the literature. It is important to consider GIST in the differential diagnosis in patients with known NF1 presenting with abdominal symptoms including GI bleeding.
CASE REPORT
This 75-year-old lady with a background of neurofibromatosis presented to the medical team with melaena and PR bleeding of both altered and fresh blood, with intermittent generalized abdominal pain. She has previously had an upper GI bleed in 2013 with upper GI endoscopy which could not find a specific lesion, and a CT colonoscopy which showed no abnormality. Examination findings revealed a soft abdomen with a palpable mass on the left-hand side. Per rectal examination revealed was soft stool only.
She had an upper GI endoscopy which was unremarkable and subsequently she was referred to the surgical team. She had a CT angiogram and continued to have PR bleeding requiring blood transfusion. She underwent surgery with an uneventful post-operative recovery and went home on the eighth post-operative day.
Investigations
Haemoglobin—88 g/l (106 g/l 2 months prior to admission)
MCV—73.4 (fl)
Haematocrit—0.278 l/l
CRP—132 mg/l
WCC—20.2 × 109/l
Erect AP chest X-ray—reported as normal
Abdominal X-ray—reported as normal
Upper GI endoscopy–hiatus hernia only
CT angiography (Figs 1 and 2)—large 9 cm exophytic cavitating lesion arising from jejunum with no evidence of active haemorrhage, with multiple further lesions in the duodenum. This was felt to be the source of the bleeding. Although the tumour was identified in this case by CT angiography, a simple contrast enhanced CT would almost certainly have demonstrated this tumour adequately, given the typical CT appearance of GIST, with significant contrast enhancement and heterogeneity.
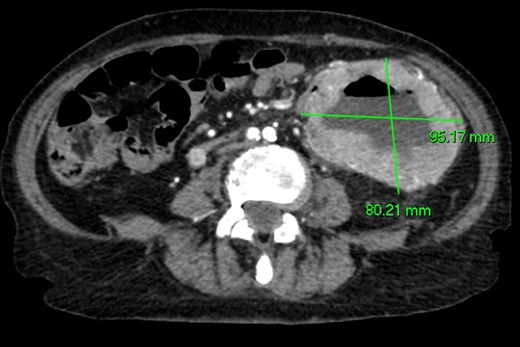
Axial CT imaging. Large exophytic cavitating lesion arising from jejunum with no evidence of active haemorrhage.
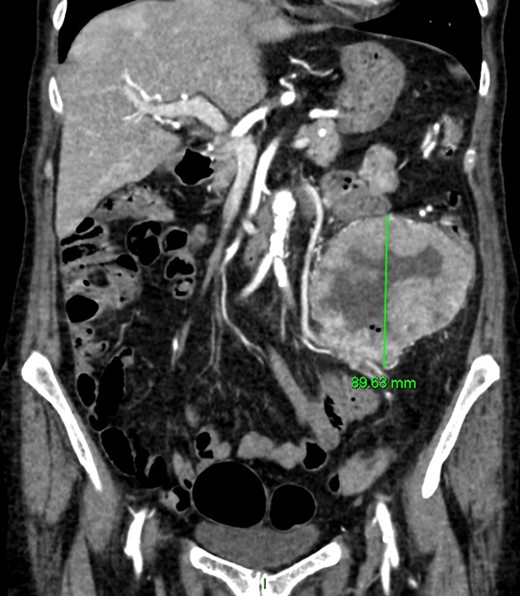
Coronal CT imaging. Large exophytic cavitating lesion arising from jejunum with no evidence of active haemorrhage.
Pathology—GIST of spindle cell type (Figs 3 and 4), 90 mm in size, with complete local excision. Mitotic count—3 per 5 mm square. CD117 (Fig. 5) and DOG1 (Fig. 6) positive staining giving a prognostic group of moderate risk (Miettinen’s classification) of progressive disease. It is widely accepted that Interstitial Cells of Cajal (ICC) are pace maker cells of the gut and probable progenitor cells of GIST. Hyperplasia of ICC can be seen in cases with NF1, however, in this case this could not be shown in the background bowel (Fig. 7).
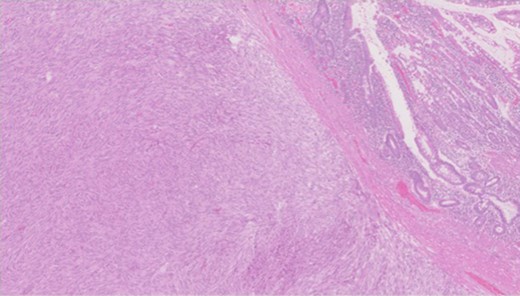
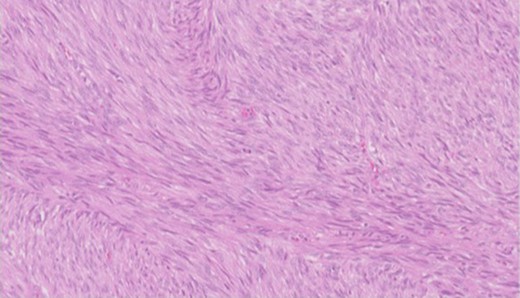
Higher power view showing the spindle appearance of tumour cells.
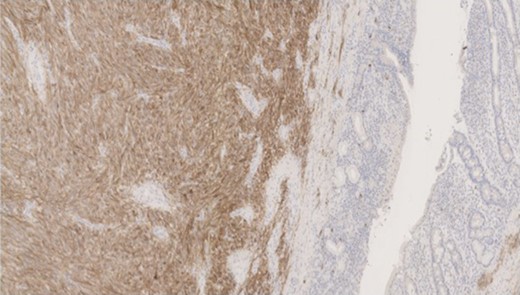
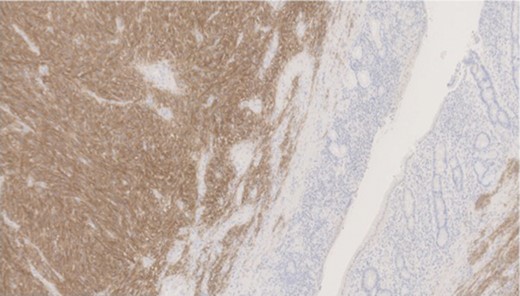

MNF staining the epithelium of the bowel while is negative in tumour cells.
Differential diagnosis
GIST
Lymphoma
Schwannoma
Leiomyoma
Treatment
4 Units packed red cell transfusion.
Laparatomy with resection of small bowel tumour and small bowel anastomosis and resection of ischaemic large bowel and large bowel anastomosis. Operative findings showed a large small bowel tumour to the left of the midline, with multiple hamartomatous lesions along the small bowel with a 6 cm ischaemic segment of large bowel which had been adherent to the tumour. Specimens were sent to pathology.
Outcome and follow up
This lady was discharged on the eighth post-operative day with follow-up in the outpatient clinic 6 weeks after discharge. Her case was discussed at the MDT which recommended moderate risk GIST follow-up, which will consist of regular clinic follow-up and imaging evaluation.
DISCUSSION
GIST are rare tumours of mesenchymal origin, most commonly located in the stomach or small intestine. Symptoms may be vague including abdominal pain, nausea, vomiting, alteration in bowel habit or GI bleeding [1]. Treatment options include surgical resection for localized lesions and neoadjuvant treatment using tyrosine kinase inhibitors for advanced disease [1]. Patients with advanced GIST in the context of NF1 do not respond well to imatinib, since the NF1 mutation appears to be the key driver, rather than KIT mutations as most commonly observed. For this reason the consensus is to avoid giving adjuvant imatinib to patients with NF1 related GIST. Unless of course an imitinib sensitive mutation (e.g. KIT exon 11) is also present, which has very rarely been described [2].
NF1 is an autosomal dominant disorder. Features include café au lait spots and neurofibromata. Prevalence is 1 in 3000 people, with half of patients having a family history and half of cases arising spontaneously. There are several associated conditions including MEN2A, MEN2B, hereditary breast cancer and GIST [3]. There are various reports in the literature of GIST in association with NF1, although little is known about the exact risk of developing GIST in NF1 patients. The prevalence of GIST in NF1 is estimated at 3.9–25%, with a median onset of 49 years, and multiple lesions can occur [1, 4]. An autopsy series has documented a GIST in one-third of NF1 patients [5], and a review paper noted that more than half of GISTs in NF1 were incidental findings compared to one in five in patients without NF1 [6].
Learning points/take home messages
GIST is a known association with NF type 1.
Presenting features may include abdominal pain, nausea, vomiting, change in bowel habit or GI bleeding so it is important to consider GIST as a differential diagnosis in patients with symptomatic NF1.
If OGD is normal in cases of GI bleeding; CT angiography should be considered to further evaluate the source.
GIST should be discussed at a specialist MDT to allow for optimum surveillance according to grade of tumour.
ACKNOWLEDGEMENTS
Mr Dominique Byrne and Dr Margaret Balsitis.
Conflict of Interest statement
None declared.



