-
PDF
- Split View
-
Views
-
Cite
Cite
M O Ongele, E Benrashid, B F Gilmore, J Schroder, M Hartwig, S Zani, Robot-assisted repair of diaphragmatic hernias following ventricular assist device implantation, Journal of Surgical Case Reports, Volume 2018, Issue 2, February 2018, rjy016, https://doi.org/10.1093/jscr/rjy016
Close - Share Icon Share
Abstract
Use of ventricular assist devices (VADs) is increasingly common, as is the need for surgeons to be familiar with the management of common complications in this population. Nonetheless, repair of diaphragmatic hernias which commonly develop following VAD implantation remains technically challenging due to intra-abdominal adhesions and the proximity of vital structures. Despite the potential benefits of improved dexterity and visualization, robotic approaches have thus far not been used to address this. We present the first two described cases of robot-assisted repair of diaphragmatic hernias in the setting of prior or current VAD placement.
INTRODUCTION
Left ventricular assist devices (LVADs) are increasingly being used to provide durable mechanical circulatory support, either as a bridge to transplant or as a destination therapy in select patients. Overall, 1451 LVADs were implanted in 2010, and the prevalence of these devices is projected to increase due to a combination of rising life expectancy and improving technology [1]. In light of this expanding utilization, the management of patients with LVADs is expected to increase in parallel and it will become more and more important for surgeons to be familiar with common complications related to LVAD placement and the management of those complications.
A number of intra-abdominal complications from LVAD placement have been well-described, including injury to abdominal organs, bowel obstruction and hernia formation [2]. Hernias through diaphragmatic defects remain difficult to recognize and manage [3]. While the initial presentation of this process is usually indolent and non-specific, the sequelae can be dramatic, with either obstructive symptoms or even significant hemodynamic derangements [4]. Laparoscopic repairs of these defects have been described, however, the proximity of either LVAD hardware or a transplanted heart to the surgical field make this approach challenging [5]. Although robotic surgery could be expected to improve this by increasing surgeon dexterity and visualization, to the best of our knowledge there are no prior reports of this approach in the literature [6]. We present herein the first two reported cases of a robotic approach to repair of LVAD-associated diaphragmatic hernias.
CASE #1
Patient #1 is a 60-year-old male with a history of gastroesophageal reflux disease and ischemic cardiomyopathy. He underwent placement of a Heartmate II LVAD in 2013 before undergoing an orthotopic heart transplant (OHT) 2 months later. After an initially uncomplicated course, he presented to the emergency department 1 year following transplant with reflux, epigastric pain and a recent 10-pound weight loss. Prior endoscopic evaluations had been normal; however, a chest X-ray was suggestive of intrathoracic bowel. A subsequent CT scan demonstrated an intrapericardial hernia containing the transverse colon (Fig. 1A–D) and the patient was taken to the operating room for a robotic diaphragmatic hernia repair.
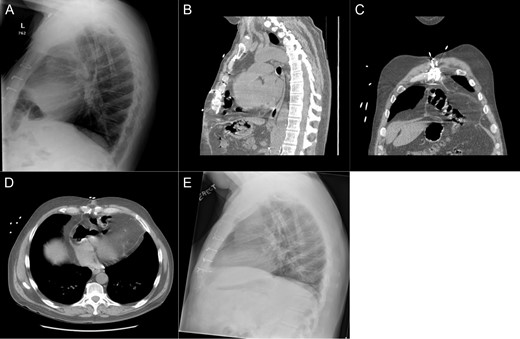
Imaging from Case #1. (A) Preoperative X-ray demonstrating loop of bowel in the thoracic cavity. (B) Preoperative CT scan, saggital view of diaphragmatic defect. (C) Preoperative CT scan, coronal view demonstrating colon passing through diaphragmatic defect. (D) Preoperative CT scan, axial view demonstrating intrapericardial transverse colon. (E) Postoperative X-ray demonstrating reduction of hernia contents.
Intraoperative assessment revealed omentum and transverse colon herniating through the left diaphragm defect (Fig. 2A). This was reduced manually without difficulty. The heart was easily seen through the defect (Fig. 2B). The area of the hiatus was evaluated noting a small hiatal hernia. The paraesophageal hernia was repaired in standard fashion with Nissen fundoplication. Attention was turned to the diaphragm defect which was closed using a running 0 permanent V-lock suture. Subsequently, a 12-cm mesh was underlaid and sewn circumferentially using 0 permanent V-lock suture (Fig. 2C). Intraoperative endoscopy demonstrated ease of passage through the GE junction into the stomach.
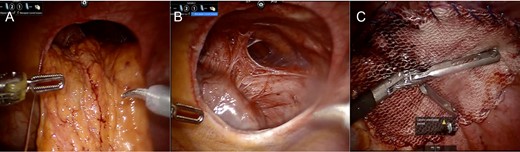
Intraoperative Images from Case #1. (A) Reduction of omentum and transverse colon through hernia defect. (B) Representative image of defect following reduction of intra-abdominal contents; heart border visible to lower left of defect. (C) Following placement of mesh for repair of diaphragmatic hernia.
The patient’s postoperative course was uneventful. Postoperative imaging showed apparent resolution of the diaphragmatic defect (Fig. 1E). At 1 month, the patient was noted to be tolerating a regular diet without complaints of any dysphagia or reflux.
CASE #2
A 43-year-old male with a past medical history significant for non-ischemic cardiomyopathy (NICM) treated with a Heartmate II LVAD and a biventricular implantable cardioverter defibrillator presented to the emergency department with new-onset change in vision, weakness of hands and numbness of upper extremities. The patient was admitted for a TIA evaluation. Due to complaints of daily vomiting a barium swallow was ordered, which demonstrated mesenteroaxial gastric volvulus. A subsequent CT scan demonstrated a diaphragmatic hernia (Fig. 3A and B) and the patient was brought to the operating room for robotic repair of the diaphragmatic hernia.
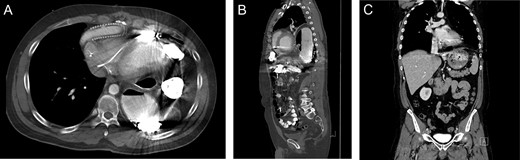
Imaging from Case #2. (A and B) Preoperative CT scan demonstrating loop of bowel in the thoracic cavity posterior to the heart and LVAD. (C) Postoperative CT scan, coronal view demonstrating repair of the diaphragmatic defect with mesh overlay.
A diaphragmatic hernia was noted immediately adjacent to the LVAD. The herniated intestinal contents were reduced without signs of visceral ischemia. A 20-cm round Parietex mesh was used to bridge the hernia defect, incorporating the LVAD device. The mesh was secured to the diaphragm using running 0-prolene suture. Postoperative course was uneventful and the patient was discharged following therapeutic anticoagulation. Postoperative imaging revealed repaired diaphragmatic defect without evidence of recurrence (Fig. 3C).
COMMENTS
This report documents a novel technique for the management of an uncommon but potentially life-threatening complication following LVAD placement or explanation. LVAD implantation requires penetration of the diaphragm by inflow and outflow cannulas at the left lateral and anterior positions, respectively, and hernias can develop at either site. The resultant hernias can be extremely complicated, due to either the immediate proximity of hardware seen in Case #2 or the extension of the herniated contents through the diaphragm into the pericardial space as seen in Case #1. The technical difficulty of safely repairing these defects is exacerbated by the potential adhesive burden resulting from intraperitoneal placement of LVAD components. Although those intra-abdominal adhesions may make cannula defect closure more difficult, limited data suggest that surgical closure at the time of LVAD explanation does decrease the risk of hernia formation from 15.9 to 4.3% [3].
Although robot-assisted repair of LVAD-associated diaphragmatic hernias is previously unreported, robotic approaches to both hiatal hernias and esophagectomy are well-described [7]. Although robot-assisted approaches are likely not necessary or appropriate for most cases of diaphragmatic hernias, the increased dexterity, improved visualization, and ergonomic benefits of robotic systems may provide a benefit for complex cases. These cases represent two such complicated cases which are nonetheless representative of the issues that can be expected in this population. In both cases large hernias within re-operative fields in extremely close proximity to either a transplanted heart of ongoing mechanical circulatory support was encountered. A laparoscopic repair would likely not have otherwise been feasible in these patients, and the robotic approach spared both patients a major open abdominal operation and a formal laparotomy. We have demonstrated that this is a safe, feasible and effective approach when performed by a surgeon with a high degree of robotic skill.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
FUNDING
None.



