-
PDF
- Split View
-
Views
-
Cite
Cite
Donald S. Mowlds, Clarence E. Foster, Hirohito Ichii, Invasive squamous cell bladder cancer of the ureterovesical junction in a renal transplant patient: a case report, Journal of Surgical Case Reports, Volume 2017, Issue 5, May 2017, rjx066, https://doi.org/10.1093/jscr/rjx066
Close - Share Icon Share
Abstract
It is well established in the literature that the incidence of malignancy is higher in transplant patients than in the general population. Risk factors and screening guidelines for transplant patients have been proposed, but are far from standardized. In this case report, we discuss the treatment course of a 73-year-old female with a history of renal tuberculosis, who developed squamous cell carcinoma at the transplant ureterovesical junction 6 years following graft placement. To our knowledge, this is the second reported case in a patient with a history of renal tuberculosis.
INTRODUCTION
The incidence of malignancy in transplant patients is higher than in the general population, ranging from 2% to 31% and approaching 50% in patients more than 20 years post-transplantation [1]. Here we present the case of a 73-year-old woman who developed squamous cell carcinoma of the bladder 6 years following renal transplant.
CASE REPORT
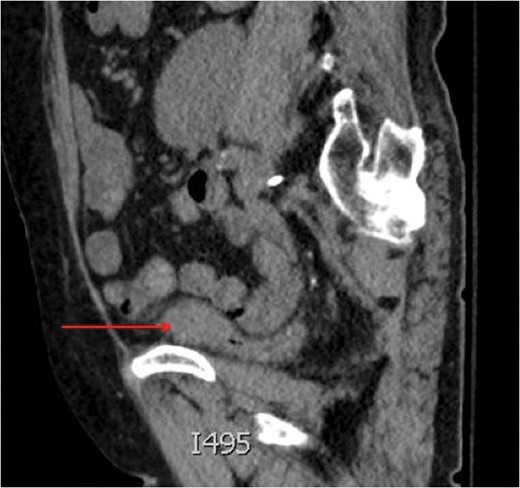
Saggital view of a mass at the dome of the decompressed bladder.
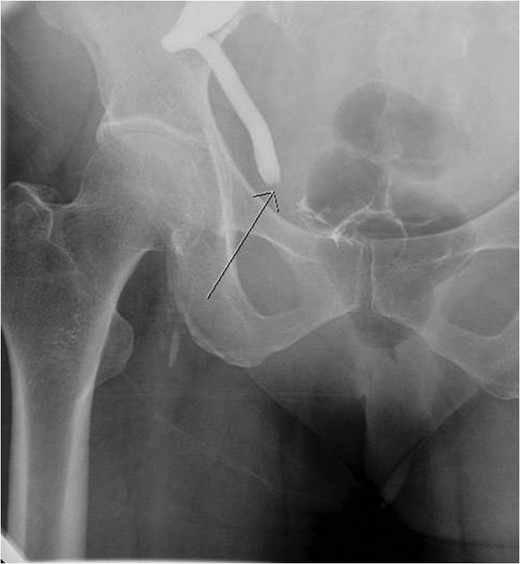
Aterograde nephrostogram demonstrating the obstruction at the ureterovesical junction.
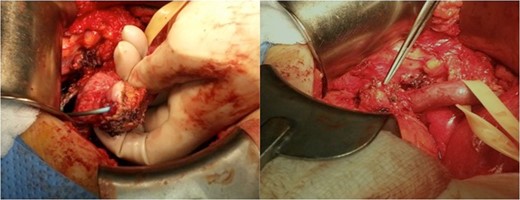
Intraoperative images depicting the tumor at the ureterovesical junction.
The patient returned to the operating room for a radical cystectomy and regional lymph node dissection with a left nephroureterectomy. The final pathology confirmed a diagnosis of invasive squamous cell carcinoma (4 cm in diameter) with invasion into the transplant ureter, perinephric fat, lamina propria and muscularis propria. Two of nine peri-vesical lymph nodes were positive for metastatic squamous cell carcinoma, making her T3aN2M0 (Stage IV). No further medical or surgical treatment was indicated for the patient. The patient was switched from Cellcept to Everolimus postoperatively to take advantage of its antitumor properties.
DISCUSSION
It is well known that the incidence of malignancy is higher among transplant recipients than the non-grafted population. Specifically, the incidence of urologic cancer is 10 times higher among renal transplant patients than in the general population [2]. Less than 5% of all bladder tumors are of squamous cell origin, with an incidence of 0.2/100 000 in the general population of Western countries [3]. Renal transplant patients have an incidence of 133/100 000, reflecting the increased risk of developing cancer in this patient population.
The association between cyclophosphamide and azathioprine therapy and the development of bladder cancer is well established [4]. This patient’s immunosuppressive therapy consisted of Cellcept and Tacrolimus. In their retrospective analysis of 3 741 consecutive renal transplants, Davis et al. discovered that 80% of their patients who developed bladder squamous cell cancer received Cellcept and Tacrolimus for immunosuppression. The remaining 20% received cyclophosphamide and azathioprine prior to the year 2000 at which time they were converted to Cellcept and Tacrolimus.
The patient discussed in this report was switched to Everolimus, an mTOR inhibitor, which has been demonstrated to reduce the occurrence of de novo cancer in kidney transplant patients [5]. This appears to be related to a suppressive effect on cell proliferation and neovascularization as well as a reduction in overall host immunosuppression [6]. In transplant recipients who have developed cancer, this appears to be an important component in the treatment strategy. In extreme cases, as with high-grade and invasive pathology, graft removal may be required so that immunosuppression may be avoided all together [7].
One study suggests screening patients with risk factors that may predispose them to the development of SCC [8]. In the single institution experience reported by Davis et al., five patients developed SCC of the bladder. Each had notable risk factors such as a history of treatment with intravenous Cyclophosphamide, chronic bladder outlet obstruction, long-term catheter use, chronic irritation to the genitourinary tract or a history of smoking. Interestingly, the patient described in this report did not possess a single one of these risk factors. European guidelines have been developed supporting the screening of renal transplant recipients with known risk factors for developing bladder cancer, most notably persistent visible or microscopic hematuria. They advocate for the use of urine cytology for non-visible hematuria (>5 red blood cells per high-power field) in the absence of urinary tract infection or nephrolithiasis [9]. Based on their experience, Davis et al. advocate that patients with even one episode of microscopic hematuria should undergo urine cytology and ultrasound studies. Cystoscopy should be performed if the previous studies are non-revealing [8]. As demonstrated by Kim et al., this strategy may result in earlier detection of bladder cancer in renal transplant patients [9]. Although the patient demonstrated neither visible nor microscopic hematuria, screening to identify potentially at risk-patients may facilitate early investigation and treatment.
There appears to be only one report in the literature describing renal tuberculosis requiring nephrectomy and the subsequent development of squamous cell carcinoma of the bladder following transplantation [10]. Whether either of these patients were at a greater risk of developing squamous cell cancer of the bladder pre-transplant is unclear, but nevertheless an interesting query for meaningful investigation.
CONFLICT OF INTEREST STATEMENT
None declared.



