-
PDF
- Split View
-
Views
-
Cite
Cite
Abbas Amirjamshidi, Seyed Abolghasem Mortazavi, Mohamad Shirani, Saeed Saeedinia, Hamed Hanif, ‘Coexisting pituitary adenoma and suprasellar meningioma—a coincidence or causation effect: report of two cases and review of the literature’, Journal of Surgical Case Reports, Volume 2017, Issue 5, May 2017, rjx039, https://doi.org/10.1093/jscr/rjx039
Close - Share Icon Share
Abstract
Coexistence of pituitary adenoma (PA) and another type of brain tumor is a very rare clinical scenario. Even though such a presentation can be an incidental event but a thorough review of the literature will be made to elucidate the possible mechanisms and treatment options in similar cases. Two cases of concomitant sellar and suprasellar/diaphragmatic tumors are reported. A 37-year-old lady with prolactinoma and a suprasellar diaphragmatic meningioma and a 42-year-old acromegalic man with suprasellar/diaphragmatic meningioma and a PA. Both meningiomas were removed transcranially. The prolactinoma could be managed medically and the growth hormone secreting adenoma was removed trans-sphenoidally. The visual problems and hormonal imbalances of both patients improved postoperatively. The literature is reviewed on this topic and the possible pathogenesis and management protocol of similar lesions are discussed.
INTRODUCTION
Meningiomas comprise 15–25% of all intracranial neoplasms [1, 2] and pituitary adenomas (PAs) are common benign neoplasms, with a prevalence of 10–23% in unselected [3, 4]. The coexistence of PA and another type of brain tumor is a very rare clinical scenario [5, 6]. The type of PAs reported in such series varied from nonfunctional to functioning adenomas [2, 5, 7–10]. In cases of PA concurrent with meningioma, GH-secreting adenoma is the most predominant [8, 9]. Even though prolactinoma represent the most common type of PA in adults (70%), the association of prolactinoma other primary brain tumors is a relatively rare occurrence [4, 5, 8].
The pathogenesis of coexistence of different lesions in the sella and suprasellar region has not been elucidated [8].
We do not intend to report collision ‘intrasellar’ pathologies but, two cases of concomitant brain tumors (CCBTs) in the sellar region are reported. Reviewing the literature for this association, we could find only five similar reports (Table 1). ‘Even though this situation can be a co-incidence’, we will discuss briefly, the ‘possible pathogenesis and management protocol’ of similar lesions.
| . | Sex, age . | PA . | Meningioma . | Treatment . |
|---|---|---|---|---|
| O’Connell 1956 | F, 47 y | Inactive intra- extrasellar | Meningothelial suprasellar | Not stated |
| Probst 1971 | M, 48 y | Cushing’s disease intrasellar | Meningothelial suprasellar | TSS for adenoma and craniotomy for meningioma |
| Wild and RIP 1974 | n.s. | Inactive | Fibroblastic | Not stated |
| n.s. | Intrasellar | Parasellar | ||
| Abs et al. 19931 | F, 82 y | Prolactinoma | Suprasellar | Subfrontal resection for meningioma, 3 months later resection tumor via TSS because decreased vision |
| F, 47 y | Inactive-intrasellar | Parasellar | ||
| TSS approach for resection of the tumor. Partial resection had been achieved | ||||
| Hainer et al. 1978 | M, 72 y | Eosinophilic | Suprasellar | Not stated |
| Bunick et al. 19785 | F, 57 y | Eosinophilic | Planum sphenoid | Both tumors was removed via a right subfrontal approach |
| Zentner 1989 | M, 46 y | Prolactinoma | Planum sphenoid | Both tumors was removed via a right sided pterional approach |
| Laun A et al. 1993 | F, 61 y | GH producing | Tuberculum sellae | Both tumors was removed via a left-sided pterional approach |
| Jaskolski DJ 1990 | M, 81 y | Inactive-intrasellar | Suprasellar meningioma | Both tumors was removed via a right subfrontal approach |
| Görge HH 1993 | M, 53 y | Prolactinoma | Para- and suprasellar | Both tumors was removed via a left-sided frontolateral approach |
| Prevedello 2007 | F, 52 y | Inactive-macroadenoma | Tuberculum sellae | Endoscopic transnasal resection of the pituitary tumor, then the planum sphenoidale was drilled and the suprasellar tumor was completely resected |
| Yu-Jen Lu et al. 2008 | F, 52 y | Inactive-intrasellar | Tuberculum sellae | Endoscopic endonasal trans-sphenoidal intrasellar tumor resection performed then Left frontotemporal craniotomy was performed for resection of meningioma |
| I. Poeata 2010 | F, 56 y | Inactive-intrasellar | Parasellar | An extended right temporo-pterional approach was used to reach access to both tumors |
| Mahvash M, et al., 2014 | F, 36 y | Inactive-intrasellar | Suprasellar | Endoscopic endonasal approach |
| Our cases | F, 37 y | Prolactinoma | Suprasellar | Craniotomy for meningioma + medication for prolactinoma Craniotomy + TSS approach |
| M, 42 y | Nonfunctional | Suprasellar |
| . | Sex, age . | PA . | Meningioma . | Treatment . |
|---|---|---|---|---|
| O’Connell 1956 | F, 47 y | Inactive intra- extrasellar | Meningothelial suprasellar | Not stated |
| Probst 1971 | M, 48 y | Cushing’s disease intrasellar | Meningothelial suprasellar | TSS for adenoma and craniotomy for meningioma |
| Wild and RIP 1974 | n.s. | Inactive | Fibroblastic | Not stated |
| n.s. | Intrasellar | Parasellar | ||
| Abs et al. 19931 | F, 82 y | Prolactinoma | Suprasellar | Subfrontal resection for meningioma, 3 months later resection tumor via TSS because decreased vision |
| F, 47 y | Inactive-intrasellar | Parasellar | ||
| TSS approach for resection of the tumor. Partial resection had been achieved | ||||
| Hainer et al. 1978 | M, 72 y | Eosinophilic | Suprasellar | Not stated |
| Bunick et al. 19785 | F, 57 y | Eosinophilic | Planum sphenoid | Both tumors was removed via a right subfrontal approach |
| Zentner 1989 | M, 46 y | Prolactinoma | Planum sphenoid | Both tumors was removed via a right sided pterional approach |
| Laun A et al. 1993 | F, 61 y | GH producing | Tuberculum sellae | Both tumors was removed via a left-sided pterional approach |
| Jaskolski DJ 1990 | M, 81 y | Inactive-intrasellar | Suprasellar meningioma | Both tumors was removed via a right subfrontal approach |
| Görge HH 1993 | M, 53 y | Prolactinoma | Para- and suprasellar | Both tumors was removed via a left-sided frontolateral approach |
| Prevedello 2007 | F, 52 y | Inactive-macroadenoma | Tuberculum sellae | Endoscopic transnasal resection of the pituitary tumor, then the planum sphenoidale was drilled and the suprasellar tumor was completely resected |
| Yu-Jen Lu et al. 2008 | F, 52 y | Inactive-intrasellar | Tuberculum sellae | Endoscopic endonasal trans-sphenoidal intrasellar tumor resection performed then Left frontotemporal craniotomy was performed for resection of meningioma |
| I. Poeata 2010 | F, 56 y | Inactive-intrasellar | Parasellar | An extended right temporo-pterional approach was used to reach access to both tumors |
| Mahvash M, et al., 2014 | F, 36 y | Inactive-intrasellar | Suprasellar | Endoscopic endonasal approach |
| Our cases | F, 37 y | Prolactinoma | Suprasellar | Craniotomy for meningioma + medication for prolactinoma Craniotomy + TSS approach |
| M, 42 y | Nonfunctional | Suprasellar |
| . | Sex, age . | PA . | Meningioma . | Treatment . |
|---|---|---|---|---|
| O’Connell 1956 | F, 47 y | Inactive intra- extrasellar | Meningothelial suprasellar | Not stated |
| Probst 1971 | M, 48 y | Cushing’s disease intrasellar | Meningothelial suprasellar | TSS for adenoma and craniotomy for meningioma |
| Wild and RIP 1974 | n.s. | Inactive | Fibroblastic | Not stated |
| n.s. | Intrasellar | Parasellar | ||
| Abs et al. 19931 | F, 82 y | Prolactinoma | Suprasellar | Subfrontal resection for meningioma, 3 months later resection tumor via TSS because decreased vision |
| F, 47 y | Inactive-intrasellar | Parasellar | ||
| TSS approach for resection of the tumor. Partial resection had been achieved | ||||
| Hainer et al. 1978 | M, 72 y | Eosinophilic | Suprasellar | Not stated |
| Bunick et al. 19785 | F, 57 y | Eosinophilic | Planum sphenoid | Both tumors was removed via a right subfrontal approach |
| Zentner 1989 | M, 46 y | Prolactinoma | Planum sphenoid | Both tumors was removed via a right sided pterional approach |
| Laun A et al. 1993 | F, 61 y | GH producing | Tuberculum sellae | Both tumors was removed via a left-sided pterional approach |
| Jaskolski DJ 1990 | M, 81 y | Inactive-intrasellar | Suprasellar meningioma | Both tumors was removed via a right subfrontal approach |
| Görge HH 1993 | M, 53 y | Prolactinoma | Para- and suprasellar | Both tumors was removed via a left-sided frontolateral approach |
| Prevedello 2007 | F, 52 y | Inactive-macroadenoma | Tuberculum sellae | Endoscopic transnasal resection of the pituitary tumor, then the planum sphenoidale was drilled and the suprasellar tumor was completely resected |
| Yu-Jen Lu et al. 2008 | F, 52 y | Inactive-intrasellar | Tuberculum sellae | Endoscopic endonasal trans-sphenoidal intrasellar tumor resection performed then Left frontotemporal craniotomy was performed for resection of meningioma |
| I. Poeata 2010 | F, 56 y | Inactive-intrasellar | Parasellar | An extended right temporo-pterional approach was used to reach access to both tumors |
| Mahvash M, et al., 2014 | F, 36 y | Inactive-intrasellar | Suprasellar | Endoscopic endonasal approach |
| Our cases | F, 37 y | Prolactinoma | Suprasellar | Craniotomy for meningioma + medication for prolactinoma Craniotomy + TSS approach |
| M, 42 y | Nonfunctional | Suprasellar |
| . | Sex, age . | PA . | Meningioma . | Treatment . |
|---|---|---|---|---|
| O’Connell 1956 | F, 47 y | Inactive intra- extrasellar | Meningothelial suprasellar | Not stated |
| Probst 1971 | M, 48 y | Cushing’s disease intrasellar | Meningothelial suprasellar | TSS for adenoma and craniotomy for meningioma |
| Wild and RIP 1974 | n.s. | Inactive | Fibroblastic | Not stated |
| n.s. | Intrasellar | Parasellar | ||
| Abs et al. 19931 | F, 82 y | Prolactinoma | Suprasellar | Subfrontal resection for meningioma, 3 months later resection tumor via TSS because decreased vision |
| F, 47 y | Inactive-intrasellar | Parasellar | ||
| TSS approach for resection of the tumor. Partial resection had been achieved | ||||
| Hainer et al. 1978 | M, 72 y | Eosinophilic | Suprasellar | Not stated |
| Bunick et al. 19785 | F, 57 y | Eosinophilic | Planum sphenoid | Both tumors was removed via a right subfrontal approach |
| Zentner 1989 | M, 46 y | Prolactinoma | Planum sphenoid | Both tumors was removed via a right sided pterional approach |
| Laun A et al. 1993 | F, 61 y | GH producing | Tuberculum sellae | Both tumors was removed via a left-sided pterional approach |
| Jaskolski DJ 1990 | M, 81 y | Inactive-intrasellar | Suprasellar meningioma | Both tumors was removed via a right subfrontal approach |
| Görge HH 1993 | M, 53 y | Prolactinoma | Para- and suprasellar | Both tumors was removed via a left-sided frontolateral approach |
| Prevedello 2007 | F, 52 y | Inactive-macroadenoma | Tuberculum sellae | Endoscopic transnasal resection of the pituitary tumor, then the planum sphenoidale was drilled and the suprasellar tumor was completely resected |
| Yu-Jen Lu et al. 2008 | F, 52 y | Inactive-intrasellar | Tuberculum sellae | Endoscopic endonasal trans-sphenoidal intrasellar tumor resection performed then Left frontotemporal craniotomy was performed for resection of meningioma |
| I. Poeata 2010 | F, 56 y | Inactive-intrasellar | Parasellar | An extended right temporo-pterional approach was used to reach access to both tumors |
| Mahvash M, et al., 2014 | F, 36 y | Inactive-intrasellar | Suprasellar | Endoscopic endonasal approach |
| Our cases | F, 37 y | Prolactinoma | Suprasellar | Craniotomy for meningioma + medication for prolactinoma Craniotomy + TSS approach |
| M, 42 y | Nonfunctional | Suprasellar |
Case 1
A 37-year-old lady presented with 8 months history of oligomenorrhea receiving LD tablet (Ovocept-LD, Aburaihan Co.) but headache, diplopia, progressive visual impairment and persistent oligomenorrhea did not improve.
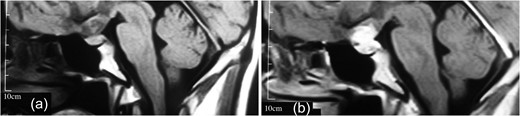
(a, b) MRI revealing a well-delineated round tumor 30 × 25 × 20 mm in diameter, T1W isointense and T2W hyperintense lesion located within the sella turcica and another dural-based lesion lying over the diaphragm sella extending to the planum sphenoidale. The intrasellar lesion showing a faint enhancement after contrast material injection but the suprasellar lesion has a bright enhancement.
Case 2
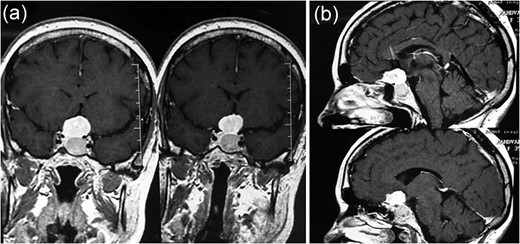
MRI showing (a) an intrasellar lesion isointense in T1W and T2W images enhancing homogenously and mildly after contrast material injection and (b) a suprasellar lesion 3 × 3 × 2 cm in diameter with the same intensity in T1W and T2W images which enhanced notably after contrast material injection.
Considering no improvement in his VA, a right pterional craniotomy was performed and a purplish, lobulated, meaty tumor uplifting the chiasm and optic nerves could be excised gross totally. The diaphragm sella and jugum sphenoidale were the areas coagulated and curetted to achieve Simpson I tumor excision followed by remarkable improvement of vision. There has been no recurrence of any of the tumors after 9 y.
DISCUSSION
Coexisting PA and suprasellar meningioma are a rare occurrence. Our search in PubMed and Google Scholar and handhold identification of the references of each article revealed 15 similar cases reported in the available literature including the five supasellar/diaphragmatic cases included in Table 1.
Considering MESH terminology, there have been different descriptions used to define coexistence of more than one tumor; ‘collision tumors’ are those with infiltration of a tumor by another type of tumor while ‘coincidental tumors’ are synchronous tumors of different histogenesis in contiguous or far from each other [7, 9]. We would like to suggest that the coexisting tumors in cases like ours are most probably of coincidental type rather collision tumors and not of the neurocutaneous disorders such as Neurofibromatosis Types I and II.
Several mechanisms have been suggested for triggering development of multiple primary brain tumors of different histology in a single patient, still the etiology remains unknown [2, 7, 9]. Some believed that their immunohisto-chemical analysis showed that concurrent adjacent double tumors occur because of activation of the signaling pathways of receptor tyrosine kinases or, one tumor may secrete a growth factor that initiates growth of another lesion [2, 7, 9]. It is yet to be investigated whether in GH-producing adenoma, might induce arachnoid cap cell transformation to meningioma [8, 9]. The other mechanisms hypothesized for the development of multiple tumors are exposure to the offending biochemical substances, genetic factors, prior trauma and surgery [7, 9].
In our first case, the serum prolactin level was 840 ng/dl and the stalk effect was almost excluded.
It is important to distinguish between an adenoma with suprasellar extension and an adenoma coexisting with a suprasellar meningioma because the treatment strategy for these tumors are different, even though no pathognomonic radiological characteristic is seen in imaging of some of these cases such as ours.
Several authors suggest that of both tumors should be removed in one session [10]. If removal of the tumors is not possible in one session, it is important to decide which tumor should be operated on first. Some suggested that avoiding the complications associated with the transcranial approaches, extended trans-sphenoidal surgery might be a good alternative choice [10].
‘The ideal surgical approach’ for treatment of such concomitant tumors is not well supported by clear evidences in the literature [1, 6, 10].
CONCLUSION
High quality imaging with good resolution and specified techniques can preclude loosing golden time for preservation of vision in these CCBT and surgical approach should be tailored according to the patient’s symptom, the anatomical characteristics of the mid skull base region and the feasibility of resection of two tumors in one session.
CONFLICT OF INTEREST STATEMENT
None declared.



