-
PDF
- Split View
-
Views
-
Cite
Cite
Vincent Chong, Jonathan Zwi, Fritha Hanning, Remy Lim, Andrew Williams, Jon Cadwallader, A case of large cell neuroendocrine carcinoma of the bladder with prolonged spontaneous remission, Journal of Surgical Case Reports, Volume 2017, Issue 5, May 2017, rjw179, https://doi.org/10.1093/jscr/rjw179
Close - Share Icon Share
Abstract
Large cell neuroendocrine carcinoma (LCNEC) of the urinary bladder are rare. We present a case of a 72-year-old man who presented with back pain and acute renal failure. Ultrasound showed a soft tissue mass in the base of the bladder causing bilateral ureteric obstruction. Subsequent biopsy of this mass demonstrated neuroendocrine carcinoma. He was commenced on neoadjuvant chemotherapy (carboplatin/etoposide) and proceeded to a radical cysto-prostatectomy. Histology revealed a LCNEC involving the bladder, T4a with invasion through to adipose tissue and posteriorly at perivesical resection margins. In addition, there was a Gleason score 9 prostatic adenocarcinoma, distinct from the neuroendocrine carcinoma. Following surgery, the patient developed gross local-regional recurrence and refused further systemic therapy. However, 1 year following referral to palliative care, a further CT-PET showed complete spontaneous remission of his disease. There are only few case reports of LCNEC of the urinary bladder therefore the pathogenesis and treatment protocol are still unclear. This case report highlights the unpredictable nature of this disease.
INTRODUCTION
Neuroendocrine carcinomas of the urinary bladder are rare. They account for <1% of bladder malignancies [1]. The majority of neuroendocrine carcinomas of the urinary bladder are small cell carcinomas. In recent years, a few cases of large cell neuroendocrine carcinomas (LCNEC) have also been reported [2].
We present a case of LCNEC of the urinary bladder which achieved complete spontaneous remission.
CASE REPORT
Mr U is a 72-year-old male, with a background of clinical T2N0M0 High Grade Urothelial Carcinoma bladder cancer treated with radical radiotherapy with neoadjuvant Cisplatin 2 years previously. He represented with back pain and acute kidney injury.
An US renal tract during this presentation showed a soft tissue mass in the base of the bladder causing bilateral ureteric obstruction. Bilateral nephrostomies were placed and his kidney function improved.
He proceeded to a biopsy of his prostate and bladder. Histology demonstrated neuroendocrine carcinoma with extensive crush artefact, with mainly small cells. His PSA was 5.9 ug/L and Chromogranin A was 214 U/L. CT Staging showed locally advanced malignancy with concern for local involvement of the mid rectum, there was no evidence of metastatic disease.
He subsequently commenced on a combination of androgen deprivation therapy and neoadjuvant chemotherapy (carboplatin/etoposide). After three cycles of chemotherapy, a PET-CT was done which showed no reduction of the large tumour volume and no metastatic disease. The patient was experiencing severe local symptoms and it was elected to go forward with radical pelvic surgery.
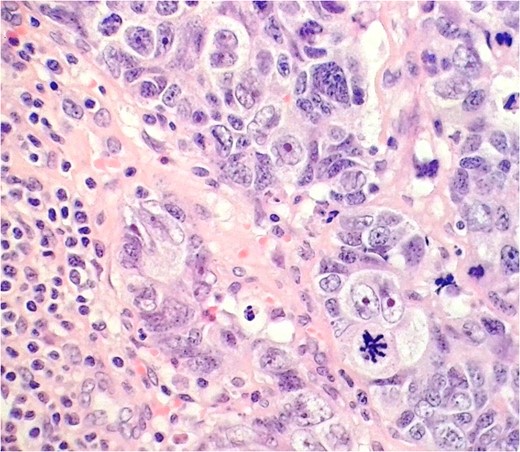
LCNEC in the external iliac lymph node. Nuclei are several times the diameter of lymphocytes (lower left), nucleoli are prominent, and chromatin quite coarse. Some nuclear moulding is seen, as in the small cell variant.
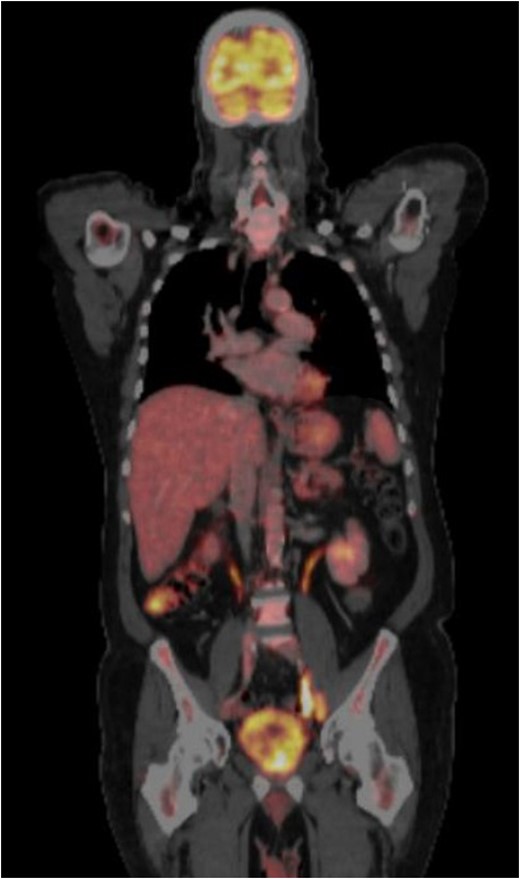
CT-PET performed after surgery showing metastatic disease with local recurrence of the mass extending into the cystectomy bed.
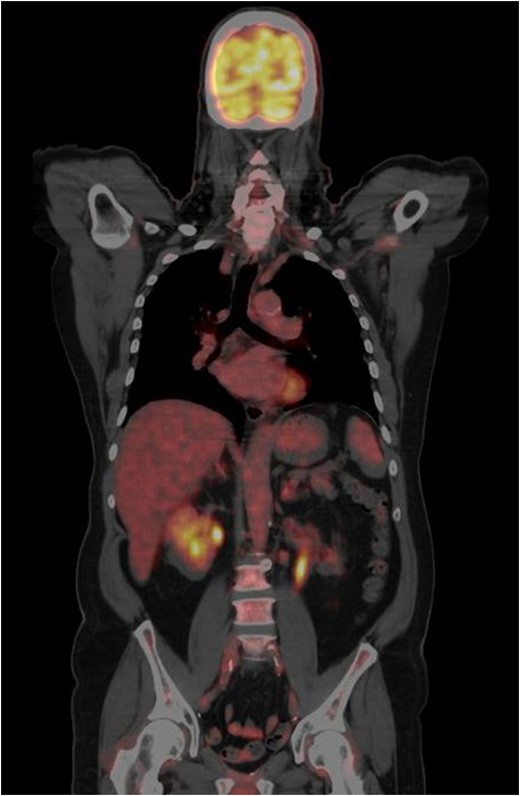
CT-PET done one year from the previous CT-PET. This showed complete remission of disease. No evidence of distant metastatic disease.
DISCUSSION
This case demonstrates a case of prolonged spontaneous remission in a patient with LCNEC in the setting of previous chemo-radiotherapy for transitional cell carcinoma. We are not aware of any cases of spontaneous remission of bladder cancer.
The most common neuroendocrine tumour of the bladder is small cell neuroendocrine carcinoma , followed by carcinoid tumours (typical and atypical), and LCNEC [3].
Large cell neuroendocrine tumour of the bladder was first described in 1986 [4] and, until now, 17 cases have been published in the international literature [3]. Most cases showed mixed histology. The most common histology is mixed with urothelial cancer, followed by adenocarcinoma, squamous cell carcinoma and sarcomatoid urothelial cancer. Only eight cases were of pure LCNEC type were reported.
In our case, our case presumably arose in a background of high-grade urothelial carcinoma, though none was found at the time of biopsy or cysto-prostatectomy.
Differential diagnosis of pure LCNEC includes metastatic LCNEC from pulmonary or gastrointestinal primary sites, local invasion of the bladder by poorly differentiated prostatic carcinoma in male patients, and primary bladder lesions such as large cell lymphomas and high-grade, undifferentiated urothelial carcinomas. These are distinguished mainly by immunohistochemistry.
The large cell variant of neuroendocrine carcinoma is considered closely related to the more common small cell variant, distinguished mainly by large total cell and nuclear size, prominent nucleoli and coarser chromatin. There is some evidence of biological differences in the two variants when occurring in the lung [5], but too few cases occur in the bladder for comparisons to be made.
We believe that the present case is the 10th reported case of pure LCNEC of urinary bladder proven post-cystectomy [2]. The previous cases are summarized in Table 1.
| Source . | Age . | Sex . | Surgery . | Adjuvant chemotherapy or radiotherapy . | Status at last follow-up . | f/u Period . |
|---|---|---|---|---|---|---|
| Hailemariam et al. [6] | 73 | M | Radical cysto-prostatectomy | Not given as immunosuppresed | Died | 2 Months after surgery |
| Lee et al. [7] | 32 | M | Partial cystectomy | Chemotherapy | Alive with lung and liver mets | 10 Months |
| Alijo et al. [8] | 40 | M | Radical cysto-prostatectomy | Chemotherapy | Alive | 13 Months |
| Alijo et al. [8] | 43 | F | Radical cysto-prostatectomy | Radiotherapy | Died | 12 Months |
| Bertaccini et al. [9] | 37 | NA | Radical cysto-prostatectomy | Chemotherapy | Alive | 22 Months |
| Lee et al. [10] | 20 | M | Partial cystectomy | Chemotherapy | Alive with lung, retroperitoneal nodal mets | 12 Months |
| Martin et al. [3] | 69 | M | Radical cystectomy | Nil | Alive | 12 Months |
| Colarossi et al. [11] | 53 | F | Cystectomy | Chemotherapy | Died | 7 Months |
| Pusiol et al. [2] | 68 | M | Radical cysto-prostatectomy | Radiotherapy and Chemotherapy | Alive with liver and bone mets | 16 Months |
| Source . | Age . | Sex . | Surgery . | Adjuvant chemotherapy or radiotherapy . | Status at last follow-up . | f/u Period . |
|---|---|---|---|---|---|---|
| Hailemariam et al. [6] | 73 | M | Radical cysto-prostatectomy | Not given as immunosuppresed | Died | 2 Months after surgery |
| Lee et al. [7] | 32 | M | Partial cystectomy | Chemotherapy | Alive with lung and liver mets | 10 Months |
| Alijo et al. [8] | 40 | M | Radical cysto-prostatectomy | Chemotherapy | Alive | 13 Months |
| Alijo et al. [8] | 43 | F | Radical cysto-prostatectomy | Radiotherapy | Died | 12 Months |
| Bertaccini et al. [9] | 37 | NA | Radical cysto-prostatectomy | Chemotherapy | Alive | 22 Months |
| Lee et al. [10] | 20 | M | Partial cystectomy | Chemotherapy | Alive with lung, retroperitoneal nodal mets | 12 Months |
| Martin et al. [3] | 69 | M | Radical cystectomy | Nil | Alive | 12 Months |
| Colarossi et al. [11] | 53 | F | Cystectomy | Chemotherapy | Died | 7 Months |
| Pusiol et al. [2] | 68 | M | Radical cysto-prostatectomy | Radiotherapy and Chemotherapy | Alive with liver and bone mets | 16 Months |
| Source . | Age . | Sex . | Surgery . | Adjuvant chemotherapy or radiotherapy . | Status at last follow-up . | f/u Period . |
|---|---|---|---|---|---|---|
| Hailemariam et al. [6] | 73 | M | Radical cysto-prostatectomy | Not given as immunosuppresed | Died | 2 Months after surgery |
| Lee et al. [7] | 32 | M | Partial cystectomy | Chemotherapy | Alive with lung and liver mets | 10 Months |
| Alijo et al. [8] | 40 | M | Radical cysto-prostatectomy | Chemotherapy | Alive | 13 Months |
| Alijo et al. [8] | 43 | F | Radical cysto-prostatectomy | Radiotherapy | Died | 12 Months |
| Bertaccini et al. [9] | 37 | NA | Radical cysto-prostatectomy | Chemotherapy | Alive | 22 Months |
| Lee et al. [10] | 20 | M | Partial cystectomy | Chemotherapy | Alive with lung, retroperitoneal nodal mets | 12 Months |
| Martin et al. [3] | 69 | M | Radical cystectomy | Nil | Alive | 12 Months |
| Colarossi et al. [11] | 53 | F | Cystectomy | Chemotherapy | Died | 7 Months |
| Pusiol et al. [2] | 68 | M | Radical cysto-prostatectomy | Radiotherapy and Chemotherapy | Alive with liver and bone mets | 16 Months |
| Source . | Age . | Sex . | Surgery . | Adjuvant chemotherapy or radiotherapy . | Status at last follow-up . | f/u Period . |
|---|---|---|---|---|---|---|
| Hailemariam et al. [6] | 73 | M | Radical cysto-prostatectomy | Not given as immunosuppresed | Died | 2 Months after surgery |
| Lee et al. [7] | 32 | M | Partial cystectomy | Chemotherapy | Alive with lung and liver mets | 10 Months |
| Alijo et al. [8] | 40 | M | Radical cysto-prostatectomy | Chemotherapy | Alive | 13 Months |
| Alijo et al. [8] | 43 | F | Radical cysto-prostatectomy | Radiotherapy | Died | 12 Months |
| Bertaccini et al. [9] | 37 | NA | Radical cysto-prostatectomy | Chemotherapy | Alive | 22 Months |
| Lee et al. [10] | 20 | M | Partial cystectomy | Chemotherapy | Alive with lung, retroperitoneal nodal mets | 12 Months |
| Martin et al. [3] | 69 | M | Radical cystectomy | Nil | Alive | 12 Months |
| Colarossi et al. [11] | 53 | F | Cystectomy | Chemotherapy | Died | 7 Months |
| Pusiol et al. [2] | 68 | M | Radical cysto-prostatectomy | Radiotherapy and Chemotherapy | Alive with liver and bone mets | 16 Months |
Bladder LCNECs have a rapid course and poor prognosis like the small cell variant. The role of surgery or chemotherapy in the treatment of LCNEC of the urinary bladder is not clear due to the rarity of the disease [1]. However, studies with pulmonary LCNEC show they respond well to standard chemotherapy regimens used for the small cell variants.
In our case, the follow-up period is 24 months following complete spontaneous remission of this very aggressive tumour and 3 years following surgical removal of the tumour. This suggests that although LCNEC is generally characterized by a very poor prognosis, it may behave very unpredictably.
CONCLUSION
LCNEC is a rare and aggressive disease. Although the overall prognosis is poor and most patient present with metastatic disease it can behave unpredictably. We believe this to be the first case of spontaneous remission of this disease. Because of the rarity of these tumours, there are no prospective trials done to evaluate the optimum treatment and our case is important as it adds to the current literature on the behaviour of this rare variant of bladder cancer.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCE
- patient referral
- ultrasonography
- back pain
- biopsy
- renal failure, acute
- adenocarcinoma
- carcinoma, neuroendocrine
- cystoscopy
- prostatectomy
- remission, spontaneous
- surgical procedures, operative
- ureteral obstruction
- adipose tissue
- urinary bladder
- histology
- palliative care
- prostate
- surgery specialty
- gleason grading system for prostatic cancer
- systemic therapy
- computed tomography/positron emission tomography imaging
- surgical margins
- chemotherapy, neoadjuvant
- carboplatin/etoposide
- large cell neuroendocrine carcinoma
- soft tissue mass



