-
PDF
- Split View
-
Views
-
Cite
Cite
Atta Nawabi, Jesus Garcia, Anna Jimenez, Scott Turner, Mojtaba Olyaee, Wei Cui, Timothy Schmitt, Sean Kumer, Mark Reintjes, Ryan Taylor, Judi Olson, Nadia Nawabi, Perwaiz Nawabi, The presence of donor liver granuloma requiring further workup to rule out parasitic disease, Journal of Surgical Case Reports, Volume 2017, Issue 4, April 2017, rjx042, https://doi.org/10.1093/jscr/rjx042
Close - Share Icon Share
Abstract
A shortage of donor organs is a major limitation to liver transplantation. Expansion of donor pool criteria to include patients with schistosomiasis diagnosed on liver biopsy might allow the allocation of more transplant livers. Schistosomiasis is a chronic parasitic disease affecting millions in endemic areas including sub-Sahara Africa that might lead to the development of granulomas as a response to the parasite’s ova and might cause chronic liver disease and portal hypertension. Due to increased mobility globally, schistosomiasis may be encountered in non-endemic areas. Currently, the usage of donor livers with known Schistosomiasis is not universally defined.
INTRODUCTION
Granulomas are focal accumulations of epithelioid cells, typically with a rim of lymphocytes and fibroblasts, representing a delayed-type hypersensitivity reaction in response to antigenic stimulation. This stimulation is usually triggered by exposure to antigen that cannot be degraded or in cases of immune dysfunction [1].
Liver granulomas have been reported in 2–15% of unselected liver biopsies [2], with the etiology varying according to geography. In the US, 75% of cases are result in sarcoidosis, mycobacterial infection, primary biliary cirrhosis and drug-induced liver injury [2]. In other countries, such as Iran, Mycobacterium tuberculosis and visceral leishmaniosis account for 60% [3].
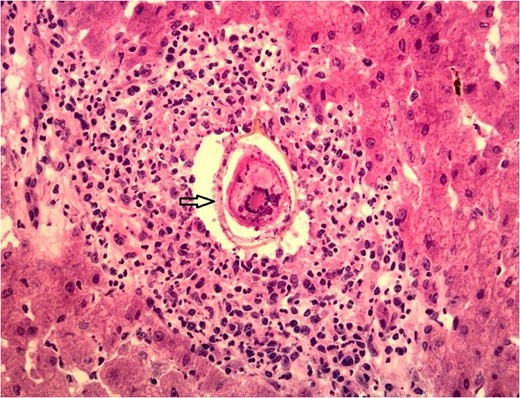
Unfortunately, liver granulomas rarely present with distinct histological features enabling pathologic diagnosis [2]. Studies have shown that 6–15% of granuloma findings are idiopathic [1, 2]. Few histological features associated with certain diseases include caseous necrosis in acid fast bacilli containing granulomas, or ova in Schistosoma mansoni [1].
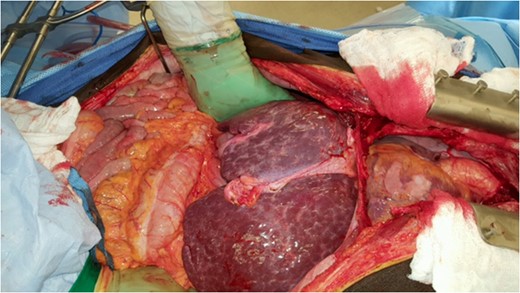
CASE REPORT
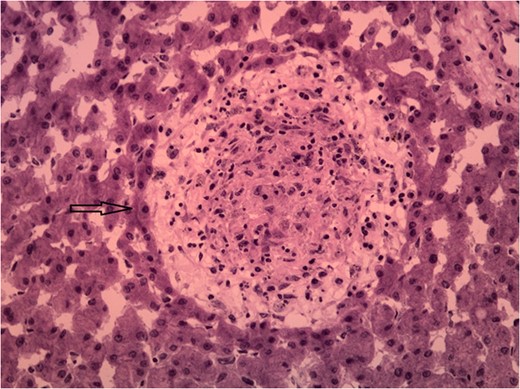
DISCUSSION
Schistosomiasis is a chronic enteral parasitic disease caused by trematode worms of the genus Schistosoma [4]. It is estimated that over 258 million people are affected yearly, with most of its disease burden in Africa, South America, the Caribbean, the Middle East and Asia [4]. At the present time, due to increased global mobility, Schistosoma may be encountered in non-endemic areas.
Over 90% of the cases of Schistosomiasis are currently found in sub-Saharan Africa, where more than 200 000 deaths occur yearly due to the disease. Schistosomiasis is the most prevalent parasitic disease in Sudan due to the wide river basin areas and large irrigated agriculture along the Nile Rivers [5].
Humans are infected when larvae in contaminated freshwater penetrate the skin. Once infection occurs, the adult worms reside in veins releasing eggs that are either shed into the environment through feces or urine, or are retained in host tissues where they induce inflammation and then die. When Schistosoma eggs are trapped in tissues, they activate an immunologic reaction leading to the development of granuloma and fibrosis [6].
Hepatic schistosomiasis leading to severe fibrosis is a well-recognized cause of chronic liver disease and portal hypertension. It results from the host’s granulomatous cell-mediated immune response to the ova antigen and progressing to irreversible fibrosis [7]. Multiple factors influence the development and pathology of Schistosomiasis including the type of immune response developed by the host, host genetic background, intensity and number of infections [8]. Most schistosomiasis is caused by Schistosoma Hematobium, S. mansoni and S. japonicum; however, S. mansoni and S. japonicum tend to cause hepatobiliary disease, while S. hematobium mainly affects the urinary tract [6]. Due to the life cycle of Schistosoma, the acute phase of infection is usually asymptomatic, the only chronic sequelae identified via tissue biopsy is necrotic eggs with granuloma formation. Thus, disease transmission is unlikely.
Due to shortages of donor organs, strategies such as living donation, split liver transplantation, domino liver transplantation and the extensive use of marginal donor grafts have been implemented in many countries. As a result of this, there are an increasing number of unusual diseases from asymptomatic donors, including schistosomiasis [9]. Reports have shown schistosomiasis transmission through liver grafts from deceased donors with no consequences for graft or recipient survival [9].
A retrospective study in Brazil highlights cases of living donor liver transplantation (LDLT) with schistosomiasis diagnosed on post-reperfusion liver biopsy showing granulomatous reaction with S. mansoni eggs in transplant recipients. Donors were treated with praziquantel. All donors had negative clinical findings for S. mansoni. Organ recipients had an uneventful post-op course [10].
Andraus et al. also describe the first intentional transplant with schistosomiasis, where known schistosoma eggs in feces were found in a donor. After treatment with praziquantel, LDLT with a graft was performed with no donor or recipient complications after 2 years post-operatively.
Chronic changes caused by schistosomiasis, leading to granuloma formation to a parasite’s egg, might be only a sign of a distant infection. Donor organs from endemic areas who have negative fecal examinations and normal liver function tests may be considered for partial liver donation. While organ shortages remains a major limitation to liver transplantation, expansion of the donor pool criteria to include patients presented in this case report might allow expansion and allocation of donor livers.
CONFLICT OF INTEREST STATEMENT
None declared.



