-
PDF
- Split View
-
Views
-
Cite
Cite
Trevor A. Teemul, Jean Perfettini, David O. Morris, John L. Russell, Post-operative avascular necrosis of the maxilla: a rare complication following orthognathic surgery, Journal of Surgical Case Reports, Volume 2017, Issue 1, January 2017, rjw240, https://doi.org/10.1093/jscr/rjw240
Close - Share Icon Share
Abstract
We present a patient with sickle cell trait who suffered avascular necrosis of the maxilla as a complication of maxillary osteotomy. Understanding the blood supply of the maxilla and how possible patient related, anaesthetic and operative factors affect it, is important in understanding how the vascularity of the maxilla can become compromised in a surgical procedure. The perioperative parameters were analysed to identify any prognostic elements. Avascular necrosis of the maxilla is a rare complication of orthognathic surgery with few cases reported in the literature. There are identifiable risk factors that can influence the blood supply of the maxilla. Careful preoperative assessment is required to exclude patient factors that have the potential to affect tissue vascularity. This in conjunction with sound anaesthetic and surgical technique should all minimize the risk of avascular necrosis. Even so it is still possible for this rare complication to occur.
INTRODUCTION
Orthognathic maxillary surgery is a safe, predictable and stable procedure. The incidence of perioperative complications is low and the number of life-threatening complications with this surgery is even lower. However, avascular necrosis of the maxilla after Le Fort I osteotomy is a rare complication that has been reported to occur in <1% of cases [1].
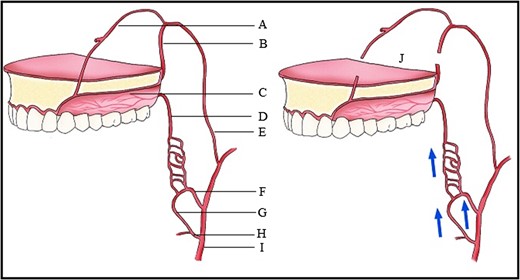
Blood supply of the maxilla. (A) Nasopalatine artery, (B) Descending palatine artery, (C) Greater palatine artery, (D) Lesser palatine artery, (E) Maxillary artery, (F) Ascending pharyngeal artery, (G) Ascending palatine artery, (H) Facial artery, (I) External carotid artery, (J) Le Fort I downfracture.
| Local . | Systemic . |
|---|---|
| Radiation treatment | Cigarette smoking |
| Infection | Pregnancy |
| Trauma | Chemotherapy |
| Surgery related | Haematological conditions |
| Sacrifice of descending palatine artery | Sickle cell disease |
| Perforation/stripping palatal mucosa | Leukaemia |
| Adrenaline injected into mucosa | Gaucher's disease |
| Perioperative vascular thrombosis | Thalassaemia |
| Segmental osteotomies | Caisson disease |
| Extensive advancement | Systemic lupus erythematosis |
| Anatomy related | Diabetes mellitus |
| Craniofacial dysplasias | Vasculitis |
| Orofacial clefts | Inflammatory bowel disease |
| Vascular anomalies | Drugs |
| Previous surgery | Vasoconstrictors |
| Cleft palate repair | High dose steroid |
| Surgically assisted rapid palatal expansion |
| Local . | Systemic . |
|---|---|
| Radiation treatment | Cigarette smoking |
| Infection | Pregnancy |
| Trauma | Chemotherapy |
| Surgery related | Haematological conditions |
| Sacrifice of descending palatine artery | Sickle cell disease |
| Perforation/stripping palatal mucosa | Leukaemia |
| Adrenaline injected into mucosa | Gaucher's disease |
| Perioperative vascular thrombosis | Thalassaemia |
| Segmental osteotomies | Caisson disease |
| Extensive advancement | Systemic lupus erythematosis |
| Anatomy related | Diabetes mellitus |
| Craniofacial dysplasias | Vasculitis |
| Orofacial clefts | Inflammatory bowel disease |
| Vascular anomalies | Drugs |
| Previous surgery | Vasoconstrictors |
| Cleft palate repair | High dose steroid |
| Surgically assisted rapid palatal expansion |
| Local . | Systemic . |
|---|---|
| Radiation treatment | Cigarette smoking |
| Infection | Pregnancy |
| Trauma | Chemotherapy |
| Surgery related | Haematological conditions |
| Sacrifice of descending palatine artery | Sickle cell disease |
| Perforation/stripping palatal mucosa | Leukaemia |
| Adrenaline injected into mucosa | Gaucher's disease |
| Perioperative vascular thrombosis | Thalassaemia |
| Segmental osteotomies | Caisson disease |
| Extensive advancement | Systemic lupus erythematosis |
| Anatomy related | Diabetes mellitus |
| Craniofacial dysplasias | Vasculitis |
| Orofacial clefts | Inflammatory bowel disease |
| Vascular anomalies | Drugs |
| Previous surgery | Vasoconstrictors |
| Cleft palate repair | High dose steroid |
| Surgically assisted rapid palatal expansion |
| Local . | Systemic . |
|---|---|
| Radiation treatment | Cigarette smoking |
| Infection | Pregnancy |
| Trauma | Chemotherapy |
| Surgery related | Haematological conditions |
| Sacrifice of descending palatine artery | Sickle cell disease |
| Perforation/stripping palatal mucosa | Leukaemia |
| Adrenaline injected into mucosa | Gaucher's disease |
| Perioperative vascular thrombosis | Thalassaemia |
| Segmental osteotomies | Caisson disease |
| Extensive advancement | Systemic lupus erythematosis |
| Anatomy related | Diabetes mellitus |
| Craniofacial dysplasias | Vasculitis |
| Orofacial clefts | Inflammatory bowel disease |
| Vascular anomalies | Drugs |
| Previous surgery | Vasoconstrictors |
| Cleft palate repair | High dose steroid |
| Surgically assisted rapid palatal expansion |
CASE REPORT
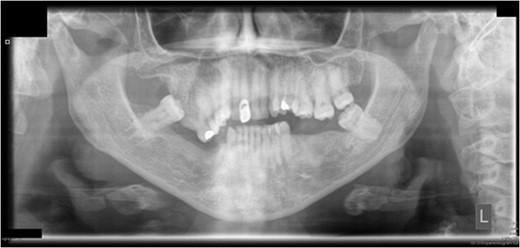
Preoperative orthopantomograph showing expansion of the right maxillary tuberosity.
The operation planned was a Le Fort I osteotomy with a right posterior segment osteotomy, which would set back the upper incisors by 6 mm, impact the upper left second molar by 4 mm and impact the posterior segment from the upper right first premolar (by 5 mm) to the upper right first molar (by 12 mm). No forward movement of the maxilla was planned. The segmental osteotomy was performed following down fracture of the maxilla and the final occlusion was aligned to a full coverage occlusal splint. The right descending palatal artery was sacrificed.
The maxillary mucosa appeared pale during the procedure, but showed capillary refill. The patient presented with wound infection and avascularity of the maxilla 1 week after the procedure. She was treated with intravenous Co-amoxiclav for 2 weeks. A course of hyperbaric oxygen treatment was organized at 14 and 18 days post-operatively. She was started on therapeutic doses of Pentoxyfylline and Vitamin E to improve capillary blood flow.
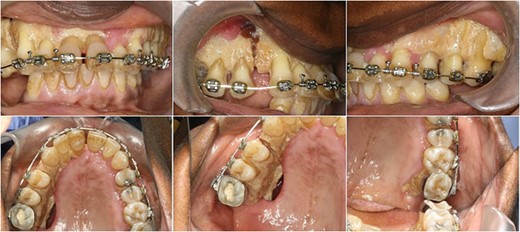
DISCUSSION
Blood flow to the maxilla is reduced by 50% in the first post-operative day after sacrifice of the descending palatine arteries [3]. However, there is excellent collateral blood supply particularly if only one artery is sacrificed, as in this case. Experimental studies have shown that loss of the descending palatine arteries results in a transient ischaemic period that is compensated for by a vascular proliferation that allows tissue healing. The collateral microvasculature from other vessels including the ascending pharyngeal and facial arteries (Fig. 1), maintains the viability of the palatine pedicle. The pedicle can withstand stretching greater than 10 mm of anterior repositioning of the maxilla.
The patient had sickle cell trait. The haemogloblinopathy screen confirmed Hb A (Adult Haemoglobin) and Hb S (Sickle Haemoglobin). The Hb F (Foetal Haemoglobin) level was <0.5% and the Hb S level was 39.4%. These parameters are typical for sickle cell trait. However, for patients in Sickle Cell crisis or those expected to be exposed to severe hypoxia, Hb S level <30% is advised [4].
Craniofacial dysplasias may represent areas of anatomical variation where the blood supply may be susceptible to disruption. Although the patient had a biopsy showing normal bone, the maxillary tuberosity was expanded and hyperplastic clinically. This in itself would not however have accounted for the degree of avascularity seen in this case.
Literature suggests that segmental osteotomy is at a higher risk of ischaemic complications [5, 6]. Necrosis of the maxilla can be minimized in the following ways:
– Divide into as few segments as possible and avoid small segments anteriorly.
– Maintain the integrity of the palatal mucosa.
– Perform sagittal segmentation in paramedian sites as the mucosa is thicker and the bone thinner than the midline.
Although hypotensive anaesthesia was not purposely utilized for the procedure, it is a technique commonly used to help maintain a bloodless surgical field [7]. A mean arterial blood pressure (MAP) 30% below a patient's usual MAP, with a minimum MAP of 50 mm Hg in American society of anesthesiologists Class I patients and a MAP not <80 mm Hg in the elderly, is suggested to be clinically acceptable [8]. It is recommended that with respect to hypotensive anaesthesia:
– It should be adjusted in relation to the patient's preoperative blood pressure rather than to a specific target pressure.
– It should be limited to that level necessary to reduce bleeding in the surgical field.
– It should be confined to that part of the surgical procedure deemed to benefit by it.
– There is little need for blood transfusion perioperatively [9].
The anaesthetic concerns include the management of perioperative hypoxia, acidosis, hypovolaemia and hypothermia.
Points to consider with this case are:
Hypoxia never occurred and is usually avoided as anaesthetised patients are always maintained with an FiO2 >0.35.
Acidosis may trigger a vaso-occlusive crisis and would affect all micro-circulation; it is easily prevented by proper perfusion and maintaining circulating blood volume.
Hypothermia was prevented by patient warming.
Hypovolaemia could be contentious, especially with hypotension.
With this patient, the mechanism of avascular necrosis could not clearly be explained. In the analysis of patient, surgical and anaesthetic factors, there seemed to be issues that may have implied a greater risk.
CONFLICT OF INTEREST STATEMENT
None declared.



