-
PDF
- Split View
-
Views
-
Cite
Cite
Mehmet Ilhan, Gizem Oner, Alpay Alibeyoglu, Gülçin Yeğen, Ali Fuat Kaan Gök, Filiz Akyüz, Fuat Bicen, Primary intestinal lymphangiomatosis of the ileum in an adult—the role of surgical approach, Journal of Surgical Case Reports, Volume 2016, Issue 8, August 2016, rjw133, https://doi.org/10.1093/jscr/rjw133
Close - Share Icon Share
Abstract
Lymphangioma is a rare benign tumor that occurs due to abnormalities occurring during lymphatic development. It is usually seen in children and young adults. The incidence of lymphangiomas in the gastrointestinal tract is very low. Here we describe the case of 43-year-old woman with lymphangioma of the ileum with infiltrative polyposis-like appearing lesions diagnosed by capsule endoscopy and treated with segmental resection of affected intestinal part with laparotomy. Lesions involving mesentery and ileum were confirmed by pathology. After surgery, the patient's symptoms improved. No further therapy was needed because of the benign manner of the lesions. Patient had no symptoms in 10 months follow-up after surgery.
Introduction
Lymphangiomatosis is a multisystem disorder. Symptoms depend on the organ system involved and can be in varying degrees according to the extent of the disease [1, 2]. Early in the course of the disease patients are usually asymptomatic, but overtime the abnormally proliferating lymphatic channels that constitute lymphangiomatosis are capable of massive expansion and infiltration into surrounding tissues, bone and organs. Because of its slow course behavior and often vague symptoms, the condition is frequently under-recognized or misdiagnosed [3]. With the widespread use of capsule endoscopy, lymphangiomas of the distal jejenum and ileum have increasingly been identified and reported. Here we report a case of lymphangiomatosis of the ileum which suspicious infiltrative polipoid lesions diagnosed by capsule endoscopy and treated with resection.
Case Report
A 43-year-old female patient was admitted to our clinic with 1-year history of weakness, swelling in leg, weight loss and recurrent upper respiratory infections. The patient had no past medical history. In this period she had lost 10% of her weight. The patient was 163 cm in height and weighed 46 kg; she appeared weak and the physical examination showed no abnormalities except 2+ pretibial edema. She had a blood pressure of 130/80-mmHg and pulse rate of 64 per minute without any cyanosis. She had symmetric thoracoabdominal breathing. Abdominal examination revealed no distension, no rebound, no ascites, no hepatosplenomegaly and normoactive bowel sounds. The complete blood cell count was in normal limits except decreased lymphocytes cells (lymphocyte = 250/mm3), and the biochemical analysis of the blood for hepatic and renal function, urine analysis and fecal occult blood test were all within normal limits only lower albumin (albumin = 2.1 g/dl) level was observed. The tumor markers for CEA and CA 19-9, celiac markers and autoimmune indicators were also normal. Upper endoscopy revealed erythematous antral gastritis. No lesions were observed on the colonoscopy and the abdominal ultrasound. No pathology was detected in echocardiography.
Ultrasonography (USG) revealed multiple anechoic cystic nodularities in the intestinal wall protruding into the fluid-filled lumen (Fig. 1). Because of any diagnose could be done by ultrasound, abdominal computerized tomography (CT) has decided to withdraw.
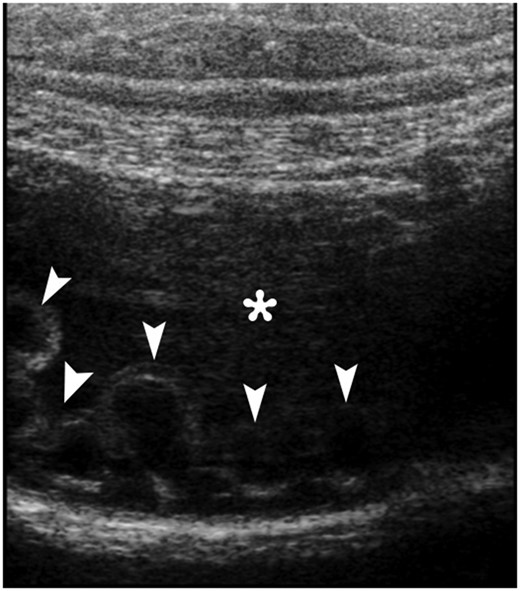
An abdominal gray-scale ultrasound image obtained with a linear transducer clearly depicts multiple anechoic cystic nodularities (arrowheads) in the intestinal wall protruding the fluid-filled lumen (*).
CT showed increased wall thickness on 40 cm segment of ileum. Capsule endoscopy was performed and a polypoid infiltrating area was found ~40–50 cm segment of middle and distal jejenum (Fig. 2A and B). Bone marrow biopsy was performed for suspicious lymphoproliferative disease. Biopsy result showed normocellular bone marrow. Flow cytometry of bone marrow mononuclear cells with the work done by immunophenotyping detected no significant antigenic exploration. It was decided to take a PET-CT for to clarify a suspicious tumor or paraneoplastic syndrome in patient. PET-CT showed diffuse wall thickness increase in the ileum. These results are in line with the assessment of the causes of protein-losing enteropathy; lymphangiectasia, intestinal lymphoma and tuberculosis. After all, surgical decision was taken with these presumptive diagnoses.
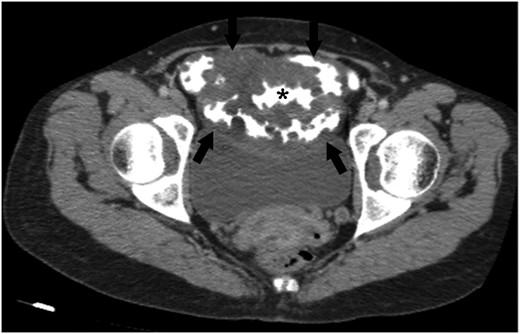
An axial CT image obtained with intravenous and oral contrast material shows abnormal wall thickening of ileal loops (arrows). Please note that the multiple nodular formations of ileal wall protruding the contrast-filled intestinal lumen (*). Abdominal CT scan also demonstrates a mass-like diffuse expansion of mesenteric root with low attenuation (arrows).
Operation started with diagnostic laparoscopic approach but nothing specific has been detected in small intestine and its meso. Then it was decided to go laparotomy. Terminal ileum was seen normal but swollen intestinal wall and pathological lymph node in the mesentery of 35–40 cm to the proximal terminal ileum were found. Excised lymph node was sent to pathology for frozen observation. Pathology result showed as lymphorrhagia. Patient was perop consulted with gastroenterology clinic and resection of the affected part of ileum decision was taken. Segmental resection including affected bowel loops were made and functional end-to-end anastomosis performed. Specimen was sent to pathology. Bowel movements are normal, and gas outlet with defecation was observed postoperatively third day. Then oral feeding was allowed on the post-op fourth day. Patient was discharged sixth day postoperatively. No further therapy was needed because of the benign manner of the lesions.
Microscopic examination of the resected specimens revealed expanded cystic vascular lesions partly extending to the intestinal mucosa in small bowel mesentery and subserosa (Fig. 3). Immunohistochemically the endothelial cells, lining the vascular structures, were reactive with D2-40, CD31 and ERG whereas negative with CD34 (Fig. 4). Present findings were found to be consistent with intestinal lymphangiomatosis.
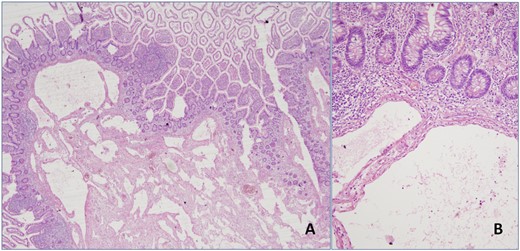
(A) Expanded cystic vascular lesion focally extending to the intestinal mucosa is seen (H&E, 4× magnification). (B) High power view of the lesion (H&E, 20× magnification).
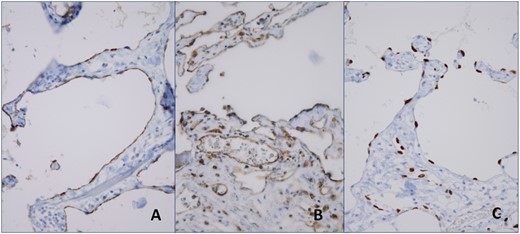
Immunohistochemically the endothelial cells, lining the vascular structures, were reactive with D2-40 (A), CD31 (B) and ERG (C).
In the control physical examination after 1 month surgery, the patient's complaints improved and pretibial edema was not seen. Laboratory values of lymphocytes, total protein and albumin were found to increase in normal values. Patient was seen without complaints on 10-month follow-up.
Discussion
Lymphangioma is a lymphatic malformation that shows benign proliferation of lymph vessels, with the characteristics of submucosal tumors covered with normal mucosa [1, 2]. Lymphangioma's histology is divided into three main categories; capillary, cavernous and cystic. The first two types were found of skin lesions, as the third is located in the abdominal or retroperitoneal. Less than 1% of lymphangiomas affects small intestine, small bowel mesentery, omentum and retroperitoneal and it is extremely rare in adults [4–6]. We reported a case of 43-year-old female patient with intestinal lymphangioma that was treated with segmental resection and end-to-end anastomosis.
The clinical symptoms of gastrointestinal lymphangiomas vary from asymptomatic to acute abdominal symptoms such as obstruction or bleeding, according to the size and the localization of the tumor [3, 7]. By the way our patient has non-specific symptoms like weakness, fatigue, weight loss, swelling in leg and recurrent upper respiratory infections. Variable clinical symptoms make it difficult to make correct diagnosis. Investigation of the diseases considered in the differential diagnosis and specific tests must be done. Also in our case, because lymphoproliferative disorders in mind in the differential diagnosis bone marrow biopsy and flow cytometry have been performed.
Endoscopic biopsy results show us, duodenal vascular congestion, active chronic gastritis and in jejenum chronic inflammation area contain focal ectatic lymphatics. But we need to evaluate distal jejenual and ileal area. Capsule endoscopy is preferable to evaluate the small intestine in detail because endoscopy remains inadequate in assessment of the small intestine [8]. About 40–50 cm long polipoid, infiltrative area and lymphangiectasia of mid-distal jejenual segment were detected in capsule endoscopy.
However, rarely occurring, intestinal lymphangioma in adults occasionally appears on CT as a solid mass and it may be confused with metastasis in patients with malignancies. Therefore, FDG PET-CT may be helpful in excluding the presence of metastasis. In our patient, FDG-PET results showed us only ileal increased diffuse wall thickness.
As well as in our patient, surgical decision can be considered when the tests are insufficient for defining the wall thickness increase in the small intestine and exact diagnosis. Laparoscopy can be the option of both diagnosis and treatment in such cases. In our case, operation was initiated by laparoscopy but the absence of pathological findings in the exploration of the small intestine and meson, and then the decision of laparotomy was taken in order to make further detailed assessment. Pathological lymph nodes and swollen intestinal segments were observed at 35 cm proximal to the terminal ileum in laparotomy, and 20-cm long segmental bowel resection with functional end-to-end anastomosis was performed. Recurrence was shown with the inadequate resections of such cases before. These patients must be closely monitored for recurrence with clinical and radiological examinations [9].
Definite diagnosis of lymphangioma is made by histopathology and immunohistochemistry. Mesothelial cells stained positive with cytokeratin, but negative with factors VIIIs. CD31 and Proxim-1 double staining is the most reliable method to show lymphangioma endothelial cells [10]. And also in our case, immunohistochemical examination of the vascular endothelial cells of the lesion showed positive D2-40, CD31, ERG and negative CD34 results. This has enabled us to make diagnosis of lymphangiomatosis.
We want to share our knowledge and experience that we have gained in this case because intestinal lymphangiomatosis is rarely encountered situation in advanced age. As in our case, it should be noted that in patients presenting with non-specific symptoms there could be pathologies that may require surgery. Although rare, intestinal lymphangiomatosis should be kept in mind in the differential diagnosis.
Informed consent was obtained from the patient, and the study followed the guidelines of the Declaration of Helsinki.
Conflict of Interest Statement
None declared.
Funding
No author or related institution has received any financial benefit from research in this study.



