-
PDF
- Split View
-
Views
-
Cite
Cite
Tongxun Li, Yuanting Zha, Liyang Bo, Douglas J. Wirthlin, Qinyi Zhang, Surgical management for retained distal embolic protection device and fractured guidewire after carotid artery stenting, Journal of Surgical Case Reports, Volume 2016, Issue 6, June 2016, rjw105, https://doi.org/10.1093/jscr/rjw105
Close - Share Icon Share
Abstract
Entrapment and fracture of carotid angioplasty and stenting hardware is a rare complication of percutaneous stenting procedures. We describe a case of a retained distal filter embolic protection device and guidewire in a 57-year-old male in Beijing, China. After unsuccessful attempts at removal via interventional methods, a second stent was deployed to secure the original hardware in situ, and the patient was discharged. He later experienced guidewire fragmentation in the carotid artery and aortic arch, with subsequent thrombus formation. We report partial removal of the guidewire and stent via carotid artery cutdown and open thoracotomy without complication. When efforts to retrieve stenting hardware are unsuccessful, it is never a suitable choice to leave them within the artery. We advocate for early surgical management of retained materials after unsuccessful carotid artery stenting. Furthermore, improved quality monitoring and assurance programs are needed to prevent such complications in the future.
Introduction
Angioplasty with stenting is a well-established alternative to carotid endarterectomy (CEA) for carotid artery stenosis [1]. The advent of proximal occlusion devices and distal filter embolic protection devices (EPD) have reduced the risk of embolic stroke [2]. However, an improperly placed stent may prevent retraction of the EPD within the capture sheath. Few cases have been reported, and no clear guidelines exist on management [3–5]. Exhaustive attempts at removal should be made, with immediate surgical conversion if unsuccessful. We report a case of retained stenting hardware left in situ, leading to subsequent complications requiring surgical intervention.
Case Report
A 57-year-old male in Beijing, China underwent carotid artery stenting via transfemoral approach for severe (>90%) asymptomatic right internal carotid artery (ICA) stenosis (Fig. 1a). A neurosurgical team at an outside facility performed the procedure using a 5-mm SpiderFXTM distal filter EPD and ev3 ProtégéTM 8 × 30 mm RX stent (Covidien; Plymouth, MN, USA). During deployment, this stent (referred to as Stent 1) inadvertently migrated cephalad and became lodged onto the EPD. Despite multiple attempts, the EPD was unable to be retrieved with the capture sheath. The physicians placed another ev3 7 × 40 mm ProtégéTM RX stent in the right ICA to compress the EPD and guidewire against the arterial wall. We refer to this as Stent 2, which was placed at the C1–C2 vertebral level, superior to Stent 1 (Fig. 1b). The patient was discharged on aspirin and clopidogrel, with the retained guidewire sutured in place at the groin. At time of discharge, the ICA stenosis had not been resolved.
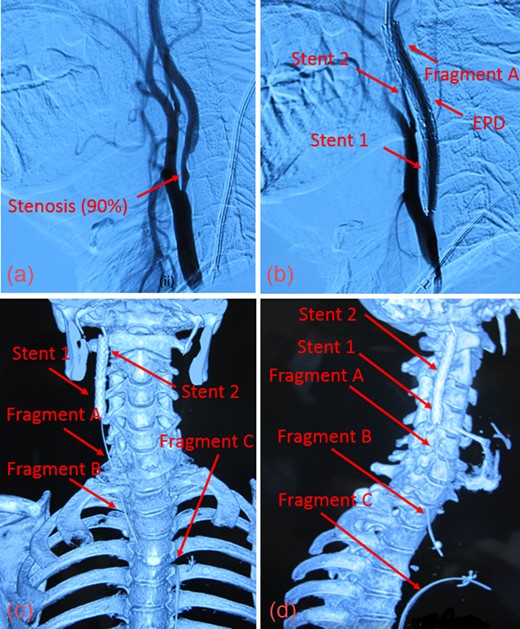
(a) Digital Subtraction Angiography of patient prior to angioplasty with 90% stenosis of the ICA. (b) Fractured guidewire when patient presented to our facility. The EPD cannot be clearly seen. (c) Reconstructed CT image in anterior view showing the 3 guidewire fragments (Fragments A, B, C) and the 2 carotid stents (Stent 1 and Stent 2) in the right ICA. (d) Reconstructed CT image in the right lateral view of guidewire fragments and stents.
One month after discharge, the patient began experiencing substernal chest pain radiating to the neck with right suborbital pain. It was discovered that the guidewire had fragmented into three pieces. He was referred to our cardiovascular surgical team at the Anzhen Stroke Center for further management.
Upon presentation, we found moderate (55%) stenosis in the right common carotid artery (CCA) and severe (90%) stenosis in the ICA inferior to Stent 1. The most superior of the three guidewire fragments (Fragment A), still attached to the EPD, was compressed by Stent 2 and extended into the CCA (Fig. 1c and d). The middle piece (Fragment B) was adhered to the right CCA wall by local fibrin deposits. The inferior fragment (Fragment C) extended from the right femoral artery to the thoracic aorta, where it punctured the proximal aortic arch wall (Fig. 2a and b). Thrombus had also formed along the entire 1-meter length of Fragment C.
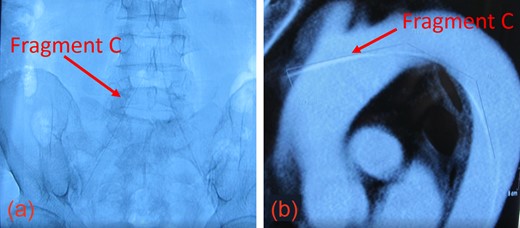
(a) The inferior aspect of Fragment C extended down to the femoral artery. (b) The superior aspect of Fragment C penetrated the thoracic aorta.
Given the risks of dislodging emboli during surgery, we initially considered employing two additional stents – one to manage the stenosis and another to compress and stabilize Fragment B. However, this would not resolve the fragmented guidewire. With the patient, we agreed upon CEA and surgical removal. Due to the extremely superior cervical location of the EPD, it was not likely to be amenable to surgical retrieval. A second surgery was scheduled for a later time to remove Fragment C.
We performed CEA at the right ICA, after which Stent 1 was cut open longitudinally and excised. Since Stent 2 was located more superiorly (C1–C2 vertebral level) and had accrued significant thrombotic material since the initial stenting procedure, we were unable to safely remove it. Thus, Stent 2 and the EDP were left intact. Fragment A was connected to the EDP and compressed against the arterial wall by Stent 2. We cut Fragment A near the stent and removed the freed portion of this fragment. Stent 2 was anchored to the artery using 7-0 Prolene sutures.
Intraoperative ultrasound confirmed the location of Fragment B in the right CCA (T1 vertebral level). We extended the CCA incision inferiorly to visualize the length of Fragment B, which was then removed intact using an artery clamp. Fig. 3a shows Fragments A and B, and Stent 1 after removal.
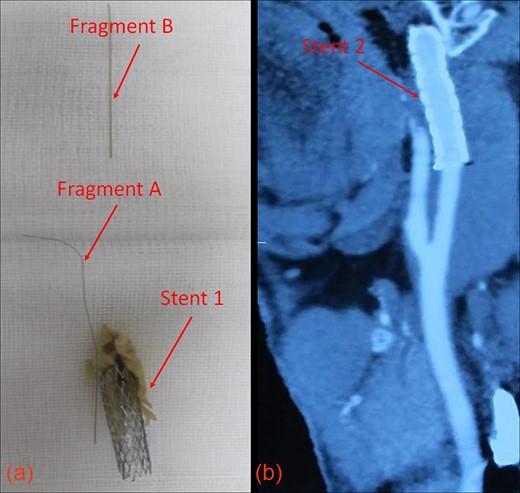
(a) Guidewire fragments and Stent 1 after removal from the patient. (b) CT image showing patent vasculature at 30 months post-operation.
The endarterectomy incision was repaired with polytetrafluoroethylene carotid patch, and intact vasculature was confirmed via fluoroscopy.
After a 1-week recovery in the intensive care unit, the patient returned to the operating room for removal of Fragment C, which was piercing the aortic arch. Via median thoracotomy, a purse-string suture was made around the point of penetration. A 6-Fr sheath was used to entrap thrombotic material as the guidewire was removed from femoral artery without incidence.
After surgery, the patient reported alleviation of his symptoms. Carotid vasculature remained patent with sufficient flow, and Stent 2 was still in place 30 months post-operatively (Fig. 3b). While the patient will require close follow-up, no complications have been reported to date, over 3 years after surgery. Informed consent was obtained from the patient prior to creation of this manuscript.
Discussion
Although rare, the risk of hardware entrapment should be considered during stenting procedures. When the EPD is irretrievable, it is not suitable to leave it in situ. Potential complications are imminent, ranging from vasospasm, thrombosis and embolism, guidewire fracture and vessel injury including carotid artery dissection [3, 6, 7].
The serious complications seen in this case were preventable. Rigorous quality assurance practices are still lacking in many low- and middle-income countries, and despite China's upper-middle income status, training and practice quality varies within the country, even in large urban settings [8, 9]. Given the patient's lack of comorbidities, a primary CEA should have been performed to treat his stenosis. Anti-platelet medications solely are not sufficient for preventing thrombosis. A surgical approach should be undertaken promptly after failed attempts to retrieve lodged hardware. Although successful interventional management has been reported, physicians must not hesitate to consider surgery if such methods prove futile [5]. A robust referral system must be available for timely transfers of patient care within and between hospitals.
Improving standard of care and patient safety needs to be a top priority. There is growing impetus for such enhanced monitoring and quality measurement systems as China undergoes major healthcare reform [10]. Bolstering regulations on reporting and establishing best practices guidelines may prevent such adverse complications in the future.
CONFLICT OF INTEREST STATEMENT
None declared.
References
Author notes
Authors contributed equally.



