-
PDF
- Split View
-
Views
-
Cite
Cite
Marcel A. Machado, Rodrigo C. Surjan, Tiago Basseres, Fábio F. Makdissi, A simple technique for hemostasis control after enucleation of deep located liver tumors or after liver trauma, Journal of Surgical Case Reports, Volume 2016, Issue 2, February 2016, rjw006, https://doi.org/10.1093/jscr/rjw006
Close - Share Icon Share
Abstract
Modern liver techniques allowed the development of segment-based anatomical liver resections. Nevertheless, there is still a place for nonanatomical liver resections. However, in some cases, there is a need for enucleation of deep located liver tumors. The main problem with enucleation of a liver tumor deeply located in the middle of the liver is the control of bleeding resulting from the rupture of small or medium vessels. The authors describe a simple way to control the bleeding without the use of any special instrument or material. This technique can also be used to control bleeding from penetrating liver injury.
INTRODUCTION
Modern liver techniques allowed the development of segment-based anatomical liver resections [1–3]. Nevertheless, there is still a place for nonanatomical liver resections [4]. Enucleation of small lesions located near the hepatic surface is achieved with low morbidity and mortality and is a current practice among surgeons. However, in some cases, there is a need for enucleation of deep located liver tumors. Another option is to use radioablation of these tumors, but this option is not always available and may result in high recurrence in inexperienced hands. The main problem with enucleation of a liver tumor deeply located in the middle of the liver is the control of bleeding resulting from the rupture of small or medium vessels. It is impossible to reach these vessels without enlarging the opening. The authors describe a simple way to control the bleeding without the use of any special instrument or material. This technique can also be used to control bleeding from stellate liver lesions after blunt or penetrating liver injury.
OPERATIVE TECHNIQUE
After identification of the lesion by inspection and intraoperative ultrasonography, Glisson's capsule is marked with eletrocautery 2 cm away from the tumor margin. The marked area is checked by ultrasonography to ascertain surgical margin right before liver transection. Hemihepatic ischemia or Pringle maneuver can be applied, according to location of the tumor. Parenchymal transection is performed with bipolar forceps or the crush technique. After removal of the tumor, a large and deep defect is produced, often with minor or major bleeding. The technique consists of filling the defect with any absorbable hemostatic tissue available, such as Surgicel (Ethicon, Cincinnati, OH, USA), and wait until this hemostatic tissue is imbibed with blood. The cautery is put at maximum power in coagulate mode and the blood is cooked until a crust is formed (Fig. 1).
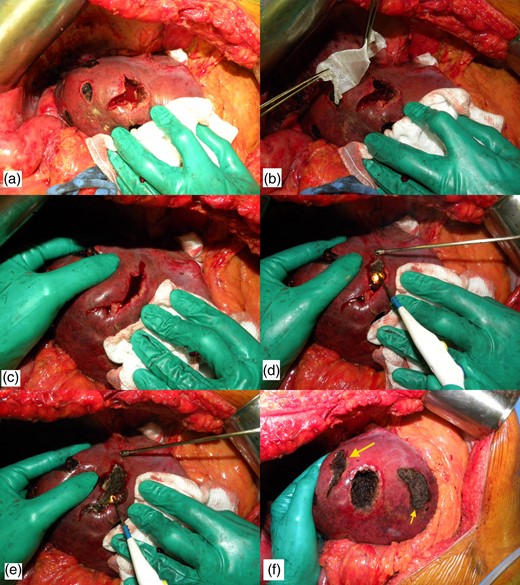
Technique of bleeding control. (a) Intraoperative photograph after enucleating a deep located metastasis. Note that there is a profuse bleeding. (b) Hemostatic tissue is inserted inside the defect. (c) Hemostatic tissue is imbibed with blood and liver borders are brought together with manual compression. (d) The cautery is put at maximum power in coagulate mode and the blood is cooked until a crust is formed. (e) The lateral compression is released and some residual bleeding is controlled with cautery. (f) Final view after the use of the technique (larger arrow). Note that another enucleated area was controlled with the same technique (smaller arrow).
DISCUSSION
Although anatomical resection, which allows a better clearance between tumor deposits and the cut section of the liver, is recommended as a standard procedure for liver tumors, nonanatomical resection is quite feasible for small tumors with little risk of microscopic local invasion [5–7]. Indeed, liver resection of peripheric small lesions is feasible and can be done with minimum morbidity and mortality rates. Enucleation is also used to remove small liver tumors from the future liver remnant after major liver resections. Enucleation of deep located liver tumors may result in rupture of small venous or portal branches that are not always easy to control. Usually, temporary compression may reduce or even stop the bleeding. However, bleeding may resume and cause postoperative bleeding. A definitive hemostasis is advised.
Another common situation that may be a good indication for the present technique is penetrating abdominal trauma with liver injury. Usually, multiple stellate liver injuries are not easy to control and liver packing may be necessary for damage control. Even radiofrequency has been suggested for bleeding control after penetrating liver trauma [8]. This technique may help dealing with these injuries without the use of any special equipment.
The present technique is feasible in every liver segment. It did not require any fancy device or instrument, and it is an important adjunct for the treatment of multiple liver tumors. We have been used this technique for hemostasis control after nonanatomical liver resections since 2012, with excellent results. This technique is useful in combination with anatomical liver resections, such as right and left hemihepatectomies, or two-stage liver resections. This technique can also be used during laparoscopic liver resections.
The described technique is quite simple, and facilitates bleeding control after liver enucleations. It is also useful to control the bleeding from penetrating liver injury and therefore can be an important tool for the general surgeon.
CONFLICT OF INTEREST STATEMENT
None declared.



