-
PDF
- Split View
-
Views
-
Cite
Cite
Elke Tieftrunk, Ihsan Ekin Demir, Helmut Friess, Güralp O. Ceyhan, Back pain as a potential indicator of local recurrence in pancreatic cancer, Journal of Surgical Case Reports, Volume 2015, Issue 10, October 2015, rjv127, https://doi.org/10.1093/jscr/rjv127
Close - Share Icon Share
Abstract
Neural invasion (NI) and severe pain are common features in patients with pancreatic cancer (PCa). Here, we present the case of a 67-year-old patient with PCa whose pre- and postoperative physical situation was clearly dominated by severe pain sensation. The resected pancreas specimen revealed severe and frequent NI by cancer cells. Seven months after R1 resection and additive chemotherapy, the patient presented with severe lumbar back pain. The CT scan showed liver metastasis and local recurrence around the celiac trunk. Yet 1 month after palliative chemotherapy, the patient presented again in poor general condition and lumbar pain requiring constant morphine intake, and died 2 days after hospitalization. Postmortem histological analysis showed local recurrence with an extensive invasion by cancer cells along almost all nerves of the celiac plexus. Hence, new-onset or recurrent back and/or abdominal pain, as in this case, should raise the clinician's suspicion for local recurrence in PCa.
INTRODUCTION
The invasion of pancreatic cancer (PCa) cells in intrapancreatic nerves (neural invasion, NI) is observed in 100% of patients with PCa [1]. Since cancer cells spread along intra- and peripancreatic nerves into the retroperitoneum, NI is critically limiting R0 resections, leading to frequent local recurrence and negatively impacting overall survival [1–3]. Furthermore, patients with severe NI have more intense pain sensation compared with patients with none or mild NI [4].
CASE REPORT
A 67-year-old male patient with severe lumbar back pain and weight loss was primarily treated in a community hospital. The preoperative CT scan showed a suspicious mass in the pancreatic head, without any evidence for extrapancreatic tumor manifestation (Fig. 1A). An explorative laparotomy was performed by the local surgeon and the tumor was classified as locally not resectable. A tumor biopsy was taken and a palliative gastroenterostomy was performed. The histopathological analysis revealed a poorly differentiated ductal adenocarcinoma of the pancreatic head. After the primary operation, the patient was referred to our center. As the tumor was judged borderline resectable, our interdisciplinary tumor board recommended ‘neoadjuvant’ chemotherapy with Gemcitabine/Oxaliplatin for 10 cycles. Restaging after the complete neoadjuvant therapy showed local tumor regression without evidence of distant metastasis. Therefore, a pancreatoduodenectomy (Whipple's operation) was subsequently performed. The histopathological examination showed an ypT3, N0 stage, Grade 3 tumor. The dorsal resection margin revealed an R1 resection, and the resected specimen revealed severe intrapancreatic NI by cancer cells (Fig. 1B). In the postoperative period, the strong pain sensations disappeared completely 2 weeks after tumor resection. With postoperative pancreatic enzyme supplementation, the patient experienced slight weight gain. At this time, an additive chemotherapy with Gemcitabine was initiated. Five months postoperatively, the patient had no pain, but suffered from mild polyneuropathy of hands and feet due to the side effects of the neoadjuvant treatment. Seven months after resection, the patient developed severe lumbar pain which again required the hospitalization of the patient. The performed CT scan showed local tumor recurrence around the inferior vena cava and liver metastasis (Fig. 1C and D). The chemotherapy regimen was changed from Gemcitabine to 5-Fluoruracil and a symptomatic adjunct analgesic therapy with Pregabalin was initiated in addition to Metamizol, which led to a short-term pain relief. However, 4 weeks later, the pain syndrome of the patient exacerbated again and was not responsive to current pain medication. Only a CT-guided blockade of the celiac plexus enabled considerable, but only temporary pain relief. Four weeks later, the patient's condition deteriorated again, with severe recurrence of uncontrollable lumbar back pain. Only a continuous morphine infusion provided a palliative pain control until patient's death.
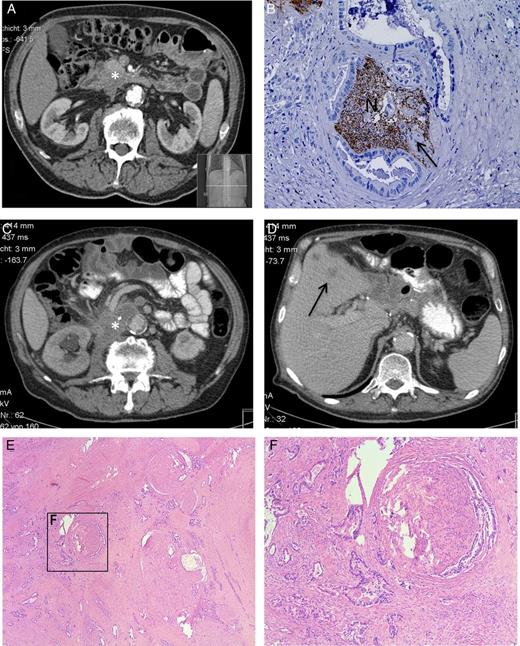
(A) Preoperative CT scan after the neoadjuvant chemotherapy shows the pancreatic head tumor (*). (B) Postoperative histopathological analysis shows severe NI of PCa cells (indicated by the arrow) in the nerve (N), staining: protein gene product 9.5 (PGP9.5), hematoxylin counter stain. (C and D) The CT scan shows the 7 months postoperative diagnosed local recurrence (*) and liver metastasis (→). (E and F) Postmortem histopathological analysis shows the invasion of pancreatic tumor cells in the extrapancreatic neural (here celiac) plexus.
Prior to the Whipple's operation, the patient gave written informed consent for an extensive postmortem histology analysis. The respected ethical approval was obtained from the ethical committee of the Klinikum rechts der Isar, Technische Universität München. This enabled us in this unique case, to correlate the symptoms of the patient with the postmortem histological samples from the peripancreatic local recurrence area. All samples from the retropancreatic region demonstrated large extrapancreatic nerves embedded in dense fibrotic tissue areas. Remarkably, nearly all visible nerves were infiltrated by very large clusters of cancer cells, and the neural structures were entirely destroyed (Fig. 1E and F). Hence, these fibrotic areas with severe NI represent the direct histological correlate of the increased retropancreatic signal density (hyperdensity) seen on the re-staging-CT scan.
DISCUSSION
The present case report not only reveals extreme pain exacerbation in close association with retroperitoneal NI in PCa, but also demonstrates the remarkable parallel between the course of pain sensation and disease progression. The initial symptoms in this patient were back pain and body weight loss, which finally led to the diagnosis of PCa. After tumor resection, the patient was initially free of pain, but re-staging was performed again due to new-onset of pain and guided to the diagnosis of local recurrence. As local recurrence of PCa is observed in ∼50% of cases with tumor relapse [5], it is crucial to know the exact localization and the initial signs and symptoms of local recurrence. While unspecific symptoms like weight loss or inappetence might be indirect symptoms for the development of local recurrence, both do not have any anatomical correlate as opposed to NI. Here, infiltration of retropancreatic nerves, which could be histologically identified in this special case, represents the radiological detectable anatomical correlate of local recurrence. Furthermore, this pathomorphological correlate is known to be associated with new-onset or recurrent back pain. Therefore, this direct association between ‘infiltration of extrapancreatic nerves’, ‘local recurrence’ and ‘back pain’ implies that this triad may aid with diagnosis of local recurrence during the course of PCa. For these reasons, we appeal for larger case controls to verify this direct association and to test the onset and the severity of ‘pain’ as a reliable indicator of local recurrence in human PCa.
CONFLICT OF INTEREST STATEMENT
None declared.
ACKNOWLEDGEMENTS
We are grateful to our patient for allowing us to learn from his case history.
REFERENCES
- morphine
- abdominal pain
- computed tomography
- back pain
- chemotherapy regimen
- patient-controlled analgesia
- autopsy
- celiac artery
- celiac plexus
- low back pain
- pain
- passive cutaneous anaphylaxis
- p-chloroamphetamine
- posterior cerebral artery
- principal component analysis
- pancreas
- sensory perception
- liver metastases
- pancreatic cancer
- palliative chemotherapy
- longitudinal relaxation rate
- tumor cells, malignant
- symptom onset



